Mutant xylose isomerase and its gene
A technology of xylose isomerase and xylose, which is applied in the field of enzyme genetic engineering and enzyme biochemical engineering, can solve the problems of less research and achieve the effect of improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Cloning of Thermus thermophilus xylA gene and mutation of Asn91 site Thermus thermophilus HB8 was purchased from American Type Culture Collection (ATCC) ATCC27634.
[0025] Culture medium preparation: Yeast powder 4g / L, peptone 8g / L, NaCl 2.0g / L, pH adjusted to 7.0, autoclaved at 121°C for 15min.
[0026] The Thermus thermophilus HB8 was inoculated in the above medium, placed on a shaker at 75° C., 200 rpm, and cultivated for 12-16 hours. 8000rpm, 4°C, centrifuge for 15min, collect the bacteria.
[0027] The genome of Thermus Thermophilus was extracted using a genome extraction kit (Shanghai Huashun Bioengineering Co., Ltd.). Using the genome as a template, use the following PCR primers (synthesized by Shanghai Boya Company):
[0028] Forward primer 5'-GGAATTCCATATGTACGAGCCCAAACCGGAGCACAGG-3' (SEQ ID NO.3)
[0029] Reverse primer 5'-AAGGAAAAAAGCGGCCGCTCACCCCCGCACCCCCAG-3' (SEQ ID NO.4)
[0030] PCR amplification of xylose isomerase gene xylA. The PCR cyc...
Embodiment 2
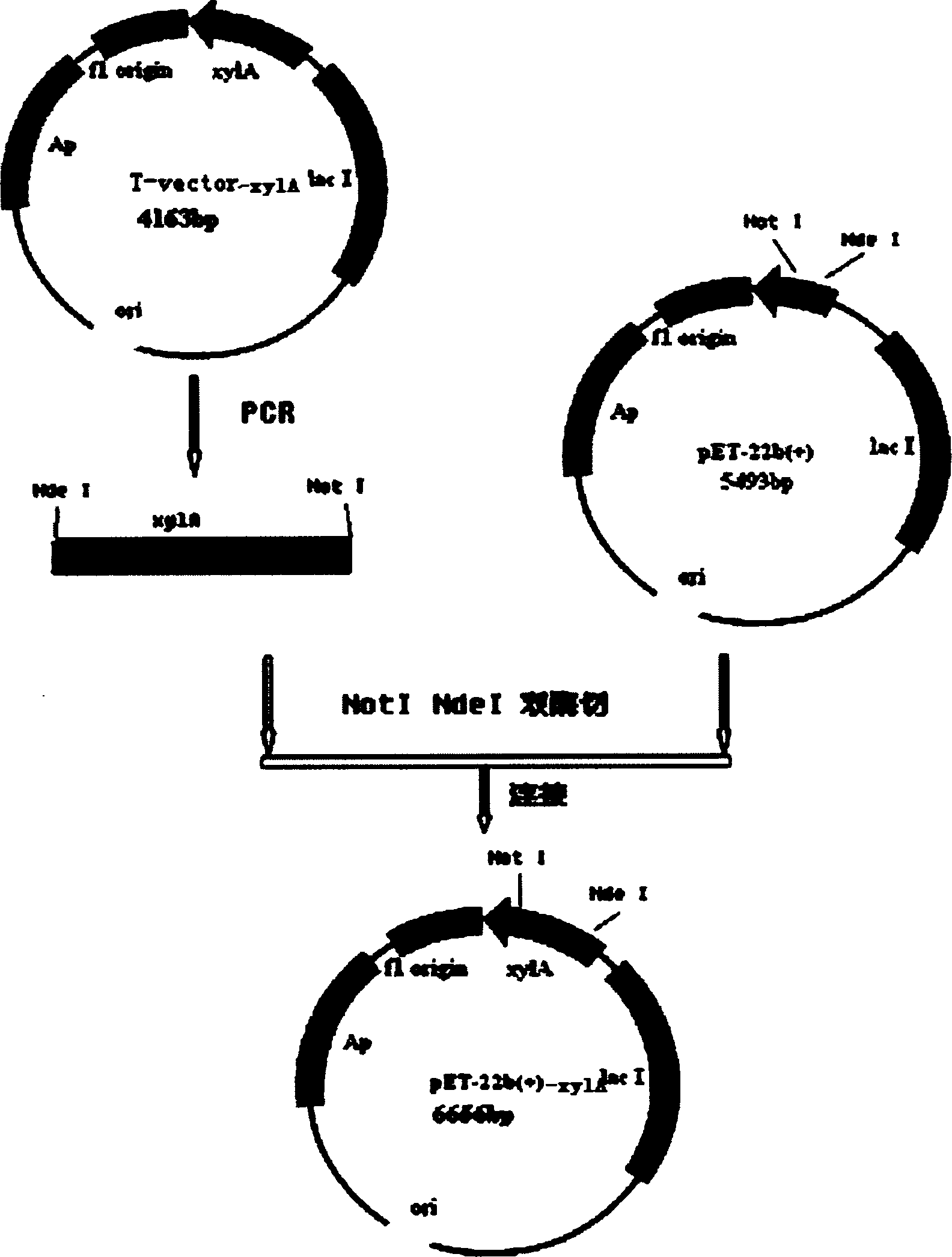
[0033] Example 2: Construction, screening and identification of expression recombinant plasmids
[0034] Forward primer 5'-CCATATGTACGAACCAAAACCGGAACATCGCTTTACCTTTT-3'(SEQ ID NO.5)
[0035] Reverse primer 5'-AAGGAAAAAAGCGGCCGCTCAACCACGCACACCCAGGAG-3' (SEQ ID NO.6)
[0036] The mutant gene xylA was amplified by PCR from the plasmid containing the mutant gene in Example 1, the mutant gene xylA and the carrier pET-22b(+) were cut with restriction endonucleases NdeI and NotI, and the above-mentioned double enzymes were digested after recovery. The mutant gene xylA and the pET-22b(+) carrier were connected, and the connection solution was transformed into E. coli DH5α competent cells by conventional genetic engineering technology, and the plasmid was extracted. The above-mentioned primers were used to amplify and identify the mutant gene xylA to be connected to the expression vector pET-22b(+).
Embodiment 3
[0037] Example 3: Expression of Thermus thermophilus xylose isomerase in Escherichia coli Rosetta
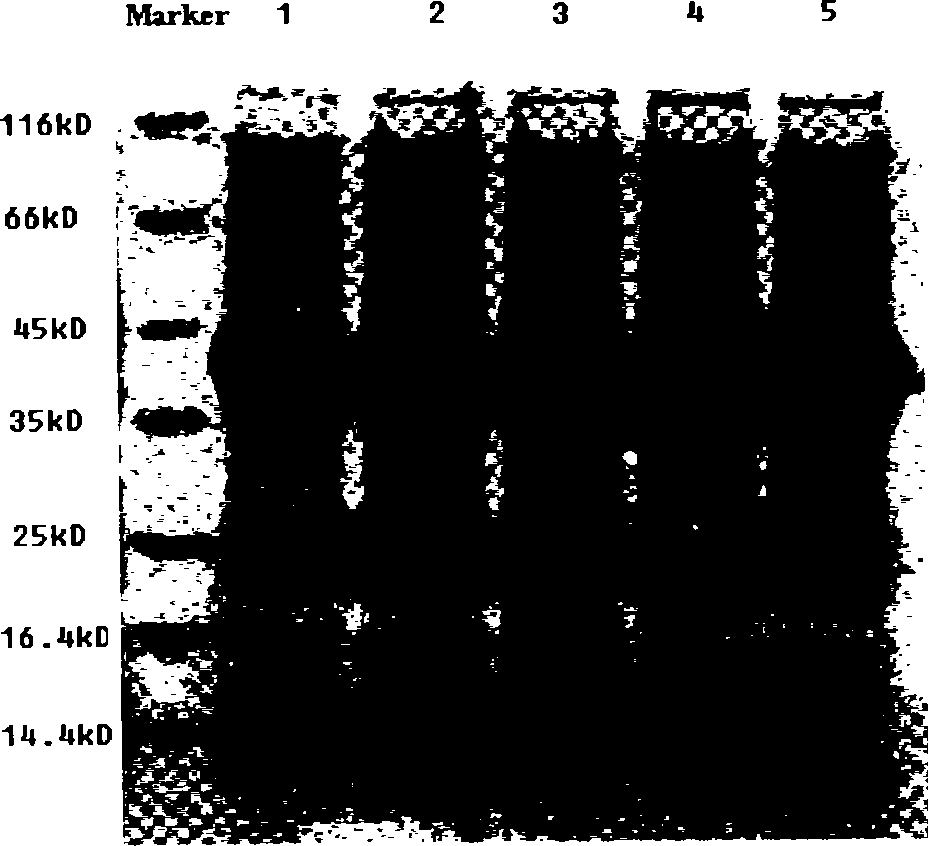
[0038] Transform the pET-22b(+)-xylA recombinant plasmid containing the mutant gene xylA into the commercialized expression host Escherichia coli Rosetta (DE3), and pick a single colony to contain 75 μg / mL ampicillin and 34 μg / ml chloramphenicol LB culture medium, cultured overnight at 37°C with shaking, then inoculated into LB culture medium containing 75 μg / mL ampicillin and 34 μg / mL chloramphenicol at 2% (v / v) inoculum size, and cultivated to OD at 37°C600 At about 0.6, add IPTG to a final concentration of 0.9 mM, induce expression for 7 hours, take a certain volume of bacterial liquid at 8000 rpm, and centrifuge at 4°C for 15 minutes to collect the bacterial cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com