A process for producing exogenous protein in the milk of trangenic mammals and a process for purifying proteins therefrom
A mammalian, transgenic technology, applied in the field of producing target protein, producing and purifying recombinant growth hormone, and manufacturing non-human transgenic mammals that produce recombinant growth hormone in their milk, can solve problems such as no reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
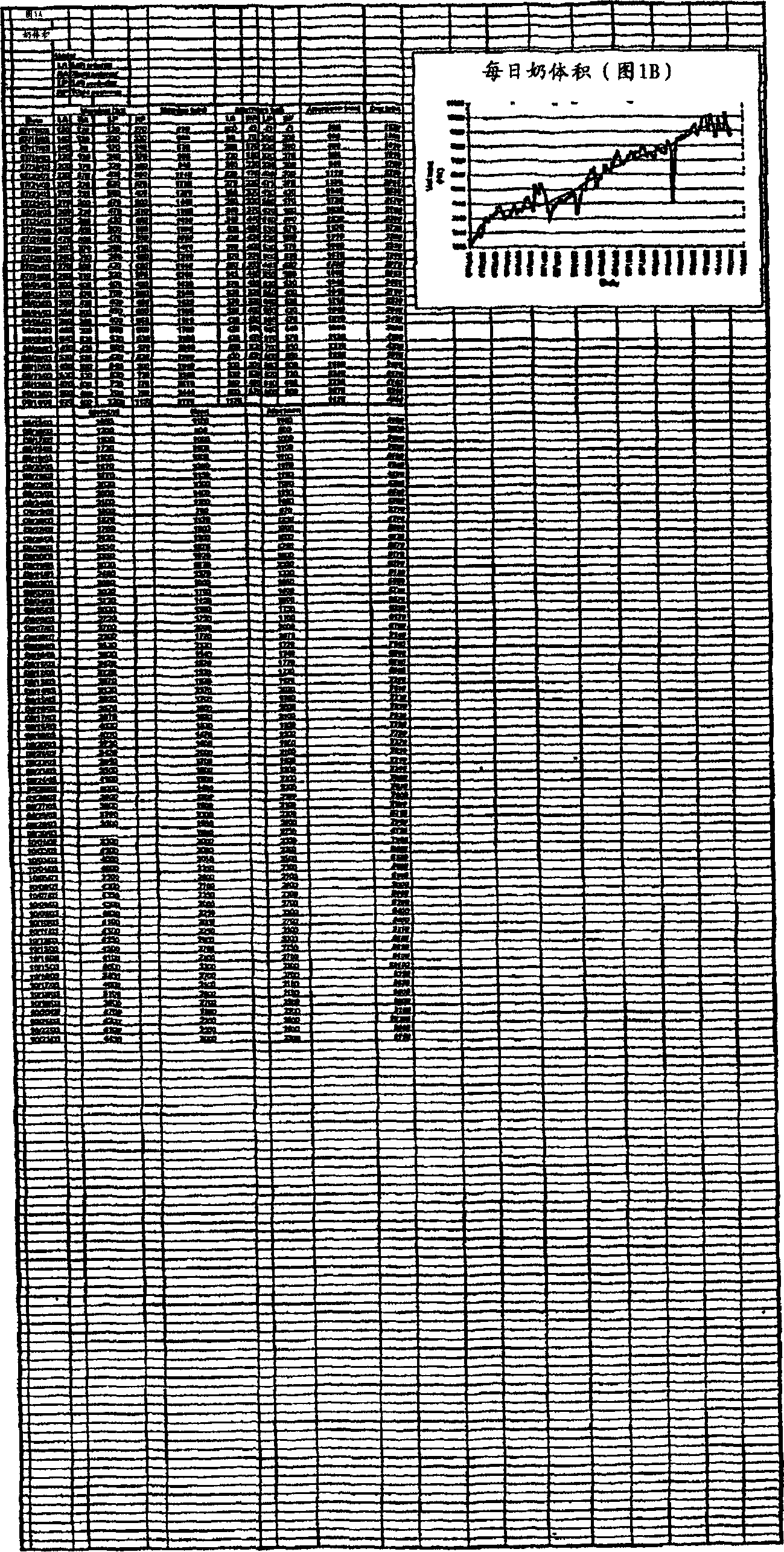
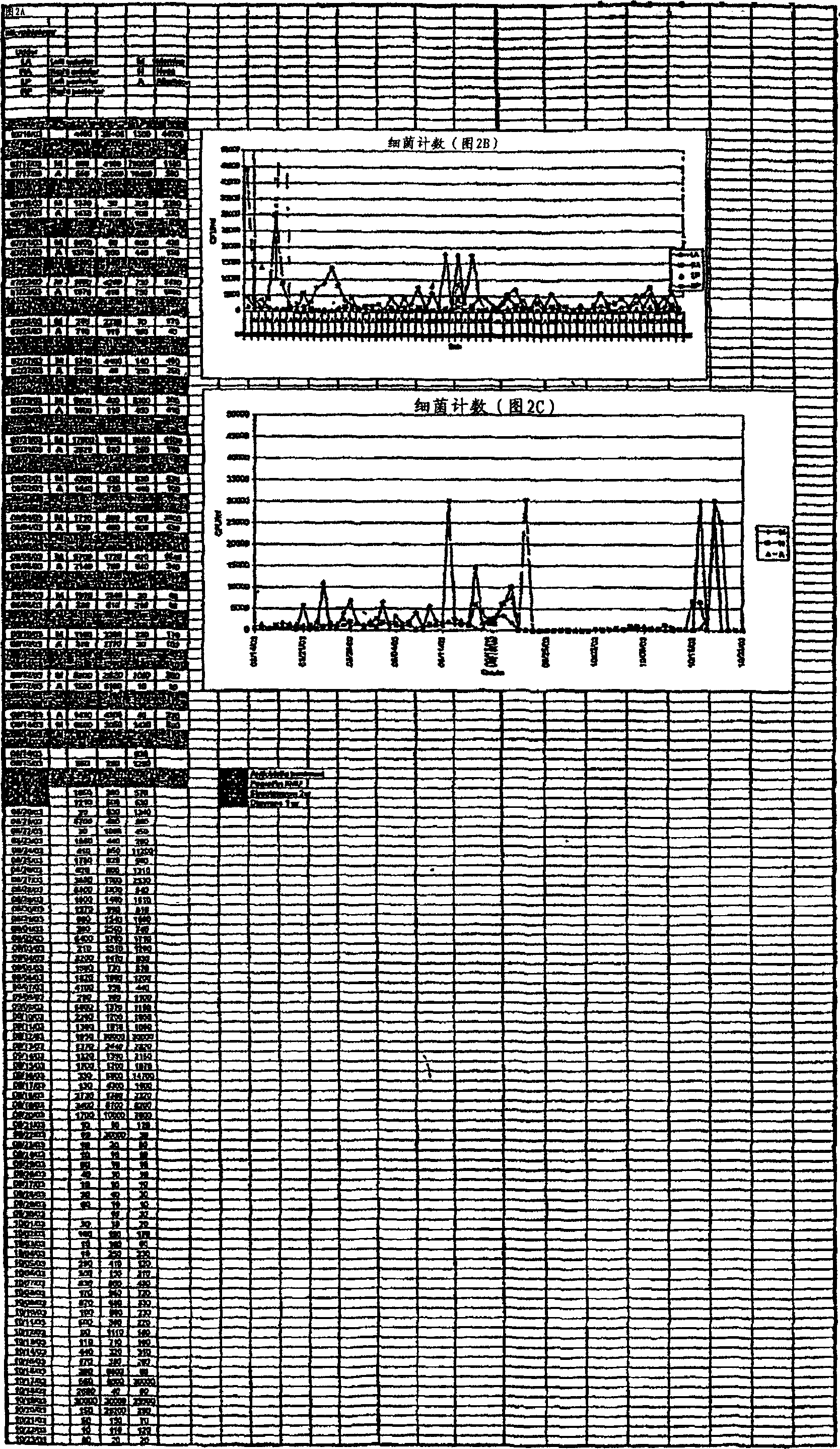
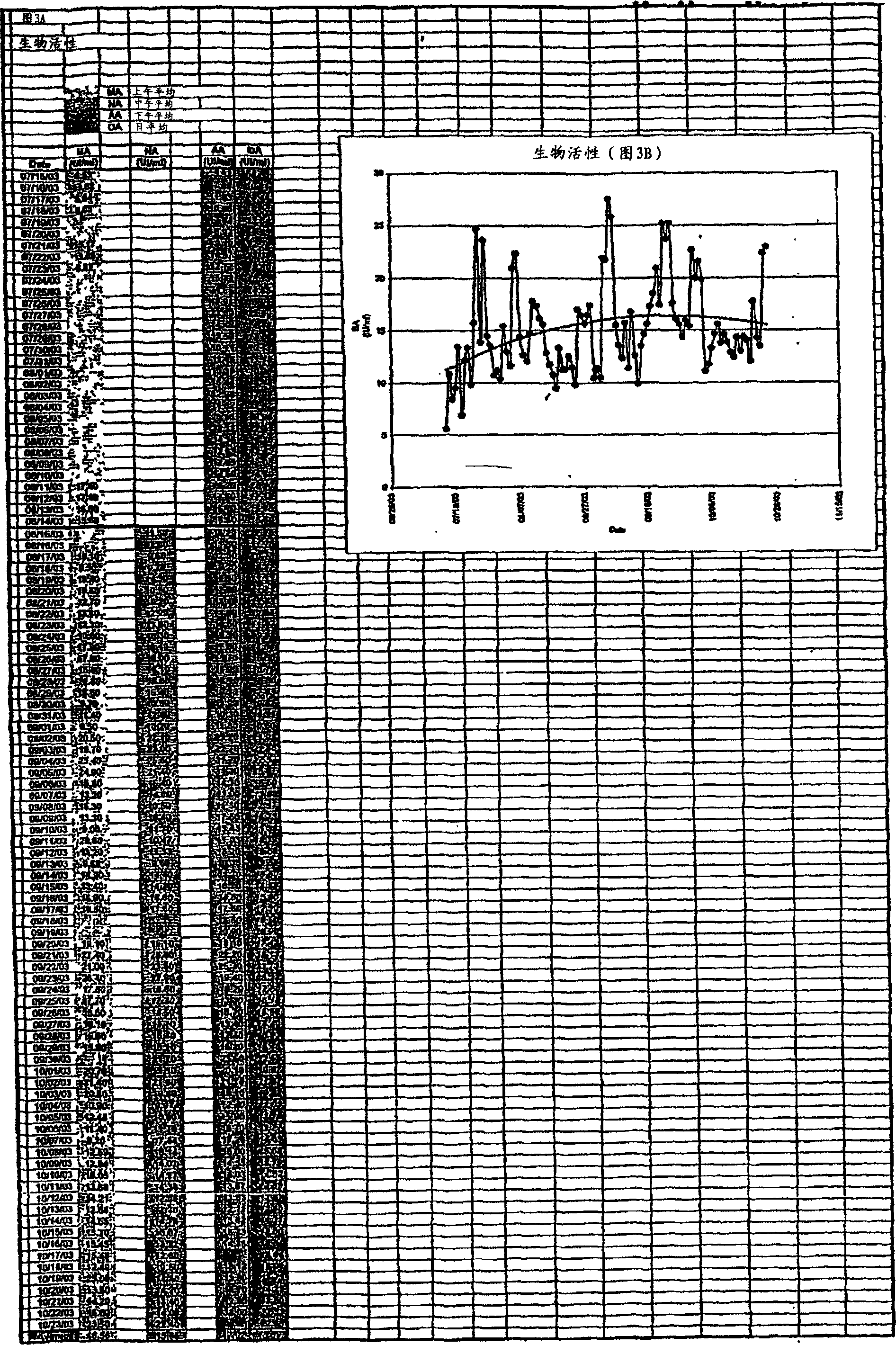
Image
Examples
Embodiment 1
[0068] Construction of expression plasmids
[0069] We generated constructs with most of the bovine β-casein gene promoter, including a short segment of the 5'-noncoding β-casein gene region, fused to the coding sequence of the human growth hormone gene. The beta casein region used in the different constructs was reduced from 3.8 kbp to about 1.3 kbp. The hGH gene comprises approximately 2 to 2.2 kbp, depending on whether an intrinsic poly A signal is included.
[0070] The expression cassette is accommodated in the polylinker of the usual cloning vectors of the pUC or pBS type.
[0071] This promoter ensures tissue-specific and developmentally regulated expression of genes under its control, such as beta casein, and in this case heterologous hGH.
[0072] The most representative plasmid is pRβhGH, which carries the full-length bovine β-casein promoter fused to the coding sequence of the human growth hormone gene.
[0073] Other constructs disclosed are...
Embodiment 2
[0099] Enucleation of oocytes and metaphase nuclear transfer in mature enucleated oocytes
[0100] Collection and in vitro maturation of bovine oocytes
[0101] Bovine oocytes were aspirated from abattoir ovaries and matured in TCM-199+5% FCS at 39°C for 24 hours. before use with CO 2 Equilibrate the maturation medium for at least 2 hours. Mature oocytes were exposed by vortexing for 2 min in warm TL-HEPES containing 1 mg / ml bovine testicular hyaluronidase.
[0102] Nuclear transfer using cumulus cells
[0103] Enucleated
[0104] Oocytes were mechanically enucleated using a Narishige hydraulic micromanipulator and a Nikon Diaphot microscope. Enucleation was performed with a 20 μm beveled and sharpened pipette. Before using 5 μg / ml bisbenzimidine (Hoechst 33342 1 ) dye to stain the oocytes for 20 minutes. Metaphases were enucleated under UV light by observing the stained chromosomes. Metaphase chromosomes were as...
Embodiment 3
[0114] Cell and Embryo Culture
[0115] Different donor cells, culture systems and oocyte recipient treatments were tested in experiments aimed at simplifying methods in bovine cloning procedures and improving embryo survival. When adult fibroblasts were used as donor cells, three culture systems for reconstituted embryos were used: TCM-199+5% FCS, Menezo+5% FCS (both containing VERO cells as a co-culture) and no co-culture. cultures but with lower O 2 concentration of SOF. SOF medium was also used to culture reconstituted embryos when the donor cells were genetically and non-genetically modified fetal fibroblasts. Finally, when genetically modified fetal fibroblasts were used as donor cells, recipient oocytes were previously treated with roscovitine(R) to arrest meiosis and optimize recipient fitness. Oocytes were aspirated from abattoir ovaries and matured in TCM-199+5% FCS at 39°C for 24 hours. For the R-treated group, oocytes were incubated with 25 μΜ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com