Bilobalide B derivatives and pharmaceutical application thereof
A technology of ginkgolides and derivatives, which is applied in the field of medicine, can solve the problems of affecting the clinical application effect, poor bioavailability, and limitation of full drug effect, and achieve bioavailability and curative effect enhancement, improve water solubility, and improve bioavailability. The effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
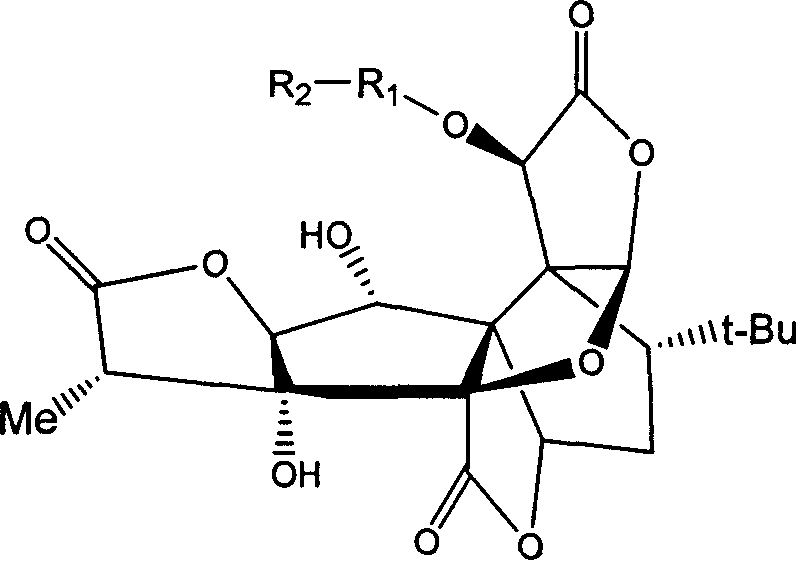
[0042] Example 1. Synthesis and structure confirmation of 10-((sodium formate) methoxy)-ginkgolide B
[0043] Add 1.47g of bromoacetic acid, 20g of potassium carbonate, and 300mg of ginkgolide B in sequence to 40mL of acetonitrile, pass inert gas, stir, and heat to reflux for 2 hours. The mixture is treated with acid solution, concentrated under reduced pressure, dissolved in chloroform, filtered, and the filtrate is concentrated. The resulting product was separated by column chromatography (eluent: chloroform / methanol=20 / 1) to obtain 142 mg of product (yield 42%), which was then dissolved in an appropriate amount of saturated sodium bicarbonate solution, adjusted to pH 9.0, and filtered to remove insoluble and the filtrate was dried to obtain the desired compound.
[0044] 1 H-NMR (DMSO-d6) δ6.22(brs, 1H), 6.05(s, 1H), 5.24(d, 1H), 4.96(s, 1H), 4.81(brs, 2H), 4.50(d, 1H ), 4.04(d, 1H), 2.81(q, 1H), 2.30(d, 2H), 2.05~1.64(m, 3H), 1.10(d, 3H), 0.96(s, 9H).
Embodiment 2
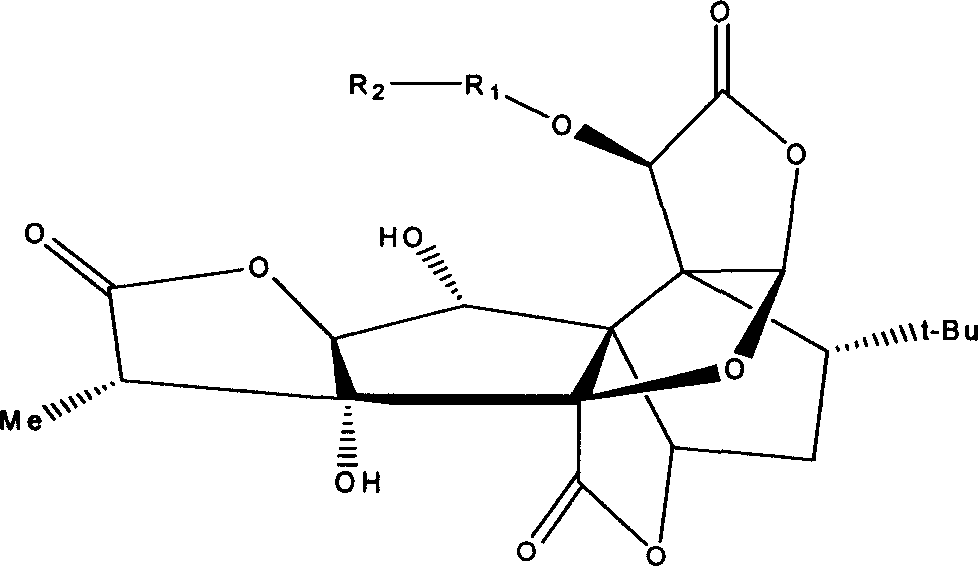
[0045] Example 2. Synthesis and structure confirmation of 10-((3'-sodium formate) propoxy) ginkgolide B
[0046] Add 1.77g of bromobutyric acid, 2.0g of potassium carbonate, and 300mg of ginkgolide B in sequence to 40mL of acetonitrile, pass inert gas, stir, and heat to reflux for 2 hours. The mixture is treated with acid solution, concentrated under reduced pressure, dissolved in chloroform, filtered, and the filtrate concentrate. The resulting product was separated by column chromatography (eluent: chloroform / methanol=20 / 1) to obtain 132 mg of the product (yield 35%), which was then dissolved in an appropriate amount of saturated sodium bicarbonate solution, adjusted to pH 9.0, and filtered to remove insoluble The filtrate was dried to obtain the expected compound.
[0047] 1 H-NMR (CDCl 3 )δ5.95(brs, 1H), 5.59(s, 1H), 5.43(d, 1H), 4.78(s, 1H), 4.70(brs, 1H), 4.46(d, 1H), 3.86(d, 1H ), 2.67(q, 1H), 2.42(d, 2H), 2.23(d, 2H), 2.01~1.55(m, 3H), 1.05(d, 3H), 0.97(s, 9H).
Embodiment 3
[0048] Example 3. Synthesis and structure confirmation of 10-((sodium formate) chloromethoxy)-ginkgolide B
[0049] Dichloroacetic acid 1.45g, potassium carbonate 2.0g, potassium iodide 400g, ginkgolide B 300mg, followed by
[0050] Add 40 mL of acetonitrile, pass inert gas, stir, and heat to reflux for 2 hours. The mixture is treated with acid solution, concentrated under reduced pressure, dissolved in chloroform, filtered, and the filtrate is concentrated. The resulting product was separated by column chromatography (eluent: chloroform / methanol=20 / 1) to obtain 161 mg of the product (yield 45%), which was then dissolved in an appropriate amount of saturated sodium bicarbonate solution, adjusted to pH 9.0, and filtered to remove insoluble The filtrate was dried to obtain the expected compound.
[0051] 1 H-NMR (DMSO-d6) δ6.42(brs, 1H), 6.15(s, 1H), 5.38(d, 1H), 5.05(ABq, 2H), 4.80(brs, 1H), 4.60(d, 1H ), 4.19 (d, 1H), 2.88 (q, 1H), 2.61 (d, H), 2.15-1.71 (m, 3H), 1.12 (d, 3H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com