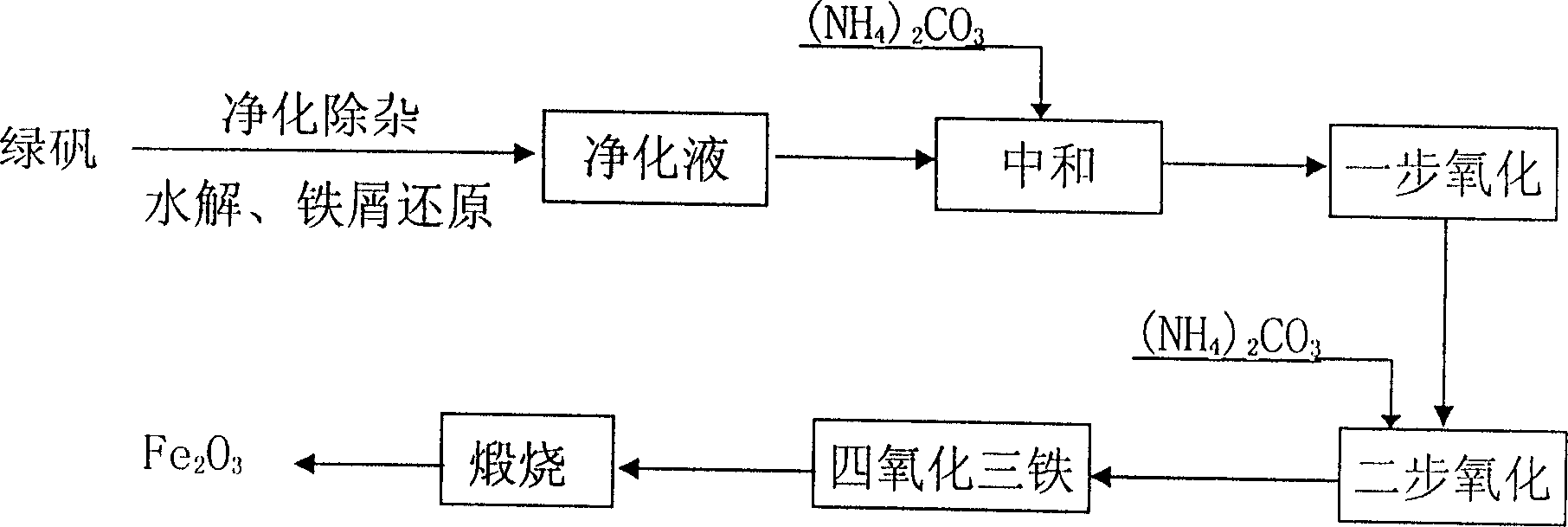
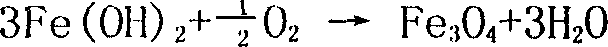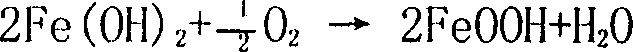Method of preparing high purity iron oxide for soft magnet using titanium white by product ferrous sulphate
A technology of ferrous sulfate and iron oxide, applied in the directions of iron oxide, iron oxide/iron hydroxide, etc., can solve the problems of inability to reduce production cost, low yield, and lack of high-magnesium raw materials, so as to solve the problems of pollution and utilization , The effect of reducing production costs and retaining product activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 2.2 liters of deionized water into a 3-liter beaker, add green vitriol 1200g while stirring, and use a small amount of chemically pure concentrated H 2 SO 4 Adjust the pH value of the solution to 2.0, add 12g of iron filings, heat to about 80°C, and keep stirring for 4 hours. Then add 350ml of deionized water to dilute and lower the temperature, then add 200ml of 0.05% polyacrylamide, stir for 2-5 minutes and then let stand for 2-3 hours. After filtration, 3L of clear and transparent green ferrous sulfate solution was obtained, and the filtrate contained Fe 73g / L. The impurity content before and after purification is shown in Table 1:
[0042] Main impurities (%)
Embodiment 2
[0044] Add 1200ml of the ferrous sulfate solution after the purification of Example 1 into a 3-liter beaker, stir at room temperature at 25°C and slowly add dropwise the neutralizing solution 500ml containing ammonium bicarbonate 23g and strong ammonia water 23ml, then heat to 60°C, Air was oxidized for 3.5 hours, and the final pH value was controlled to be 4.5. Then add dropwise the neutralizing solution 800ml containing 100g ammonium bicarbonate and 100ml strong ammonia water while oxidizing, the oxidation time is 6 hours, the terminal pH value is 6.8, stop oxidation and heating, continue to stir for 30 minutes and leave standstill for 2 hours. Then filter, wash with deionized water to wash with 1% BaCl 2 Solution detects no SO 4 2- The precipitate was baked in an oven at 100°C for 2 to 3 hours, and then moved to a muffle furnace for calcination at 680°C for 2 hours to obtain 115g of iron oxide. The Fe yield was 91.9%. The sample analysis results are shown in Table 2 [Mi...
Embodiment 3
[0046] Add 1200ml of the ferrous sulfate solution purified in Example 1 into a 3-liter beaker, heat to 60°C, stir and slowly add 550ml of neutralizing solution containing 30g of ammonium bicarbonate and 30ml of concentrated ammonia water dropwise, heat up to 70°C, and pass Air oxidation was carried out for 3.5 hours, and the final pH value was controlled at 4.0. Add dropwise 750ml of neutralizing solution containing 98g of ammonium bicarbonate and 98ml of strong ammonia water while oxidizing, the oxidation time is 7 hours, and the terminal pH value is 7.5. Stop oxidation and heating, stir for 30min and let stand for 2 hours. Then filter and wash with deionized water until SO-free 4 2- The precipitate was dried in an oven at 100°C for 2-3 hours, and then moved to a muffle furnace for calcination at 720°C for 2 hours to obtain 114g of iron oxide product with a Fe yield of 91.1%. See Table 2 for sample analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com