Haloalkyl containing compounds as cysteine protease inhibitors
A technology of halogenated alkyl and compounds, which is applied in the field of cysteine proteases, and can solve problems such as increased expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
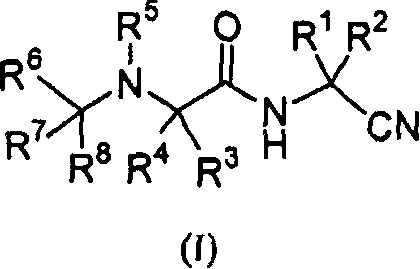
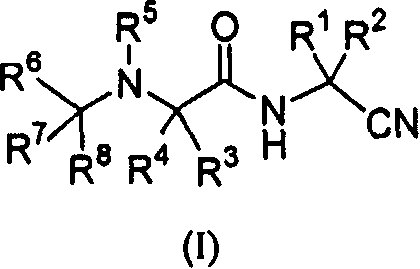
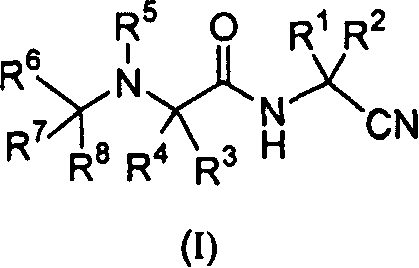
[0138] Certain compounds of formula (I) within the broad scopes and preferred embodiments set forth in the Summary of the Invention are preferred. For example:
[0139] A. A preferred group of compounds wherein R 1 and R 2 those for hydrogen.
[0140] B. Another preferred group of compounds is that wherein R 1 and R2 together with the carbon atoms to which they are attached are optionally replaced by one or two R b Those of substituted cycloalkylene, R b independently selected from alkyl, halo, dialkylamino, aryl, aralkyl, cycloalkyl, cycloalkylalkyl, heteroaryl, heteroaralkyl, alkoxycarbonyl, or aryloxycarbonyl. Preferably, R 1 and R 2 Together with the carbon atom to which they are attached, form cyclopropylene, cyclobutylene, cyclopentylene, or cyclohexylene optionally substituted by the groups described immediately above. More preferably, R 1 and R 2 Together with the carbon atoms they are attached to form cyclopropylene, cyclobutylene, cyclopentylene, cyclohexyl...
Embodiment A
[0373] Synthesis of 2(RS)-benzyloxycarbonylamino-4(RS)-(2-methoxyphenyl)pentanoic acid
[0374]
[0375] To d,l-2-methoxy-α-methylbenzyl alcohol (0.5 g, 3.29 mmol) was added 48% aq. HBr (2 mL) and the reaction mixture was stirred rapidly for 1.5 hours. Dilute the reaction mixture with n-hexane (30mL), wash with water, MgSO 4Dry, filter, and evaporate in vacuo. Crude d,l-2-methoxy-α-methylbenzyl bromide was added to tributyltin hydride (0.67 mL, 2.49 mmol), Z-dehydroalanine methyl ester (0.25 g, 1.06 mmol), and 2,2'-azobisisobutyronitrile (15 mg, 0.09 mmol) in benzene (5 mL). The reaction mixture was heated at 80° C. for 5 hours under nitrogen atmosphere. Benzene was removed in vacuo and the residue was dissolved in methanol (20 mL). 2N KOH (5 mL) was added and the mixture was stirred rapidly at room temperature overnight. Methanol was removed in vacuo and the residue was diluted with water (20 mL). The aqueous solution was washed with ether to remove the tin by-produc...
Embodiment 1
[0476] N-(1-cyanocyclopropyl)-3-phenylmethanesulfonyl-2(R)-(2,2,2-trifluoro-1(RS)-thiophen-2-ylethylamino)propane Amide synthesis
[0477]
[0478] step 1
[0479] To a cold (0 °C), stirred solution of 2(R)-amino-3-phenylmethanesulfanylpropionic acid (commercially available) (4.01 g, 19.0 mmol) in methanol (100 mL) was introduced HCl gas 15 min, the reaction mixture was sealed and stirring continued at room temperature overnight. The solvent was then evaporated under vacuum to give methyl 2(R)-amino-3-phenylmethanesulfanylpropionate hydrochloride (4.98 g) in quantitative yield.
[0480] step 2
[0481] 2(R)-Amino-3-phenylmethanesulfanylpropanoic acid methyl ester hydrochloride (4.95 g, 18.9 mmol) containing p-toluenesulfonic acid (0.19 g) and Trifluoroacetaldehyde methyl hemiacetal (3.12 g, 24.0 mmol) mixture overnight. The reaction mixture was diluted with ethyl acetate (100 mL), washed with 100% sodium bicarbonate, and then washed with water. Dry the organic phase (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com