Luminescent organic-polymer/metal complex, luminescent organic-polymer/metal complex composition capable of forming film by wet process, and process for producing the same
A metal complex, organic polymer technology, applied in electroluminescent light sources, chemical instruments and methods, luminescent materials, etc., can solve the problems of low efficiency of electroluminescent elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0044] Synthesis example 1 (polyQ 1 Synthesis)
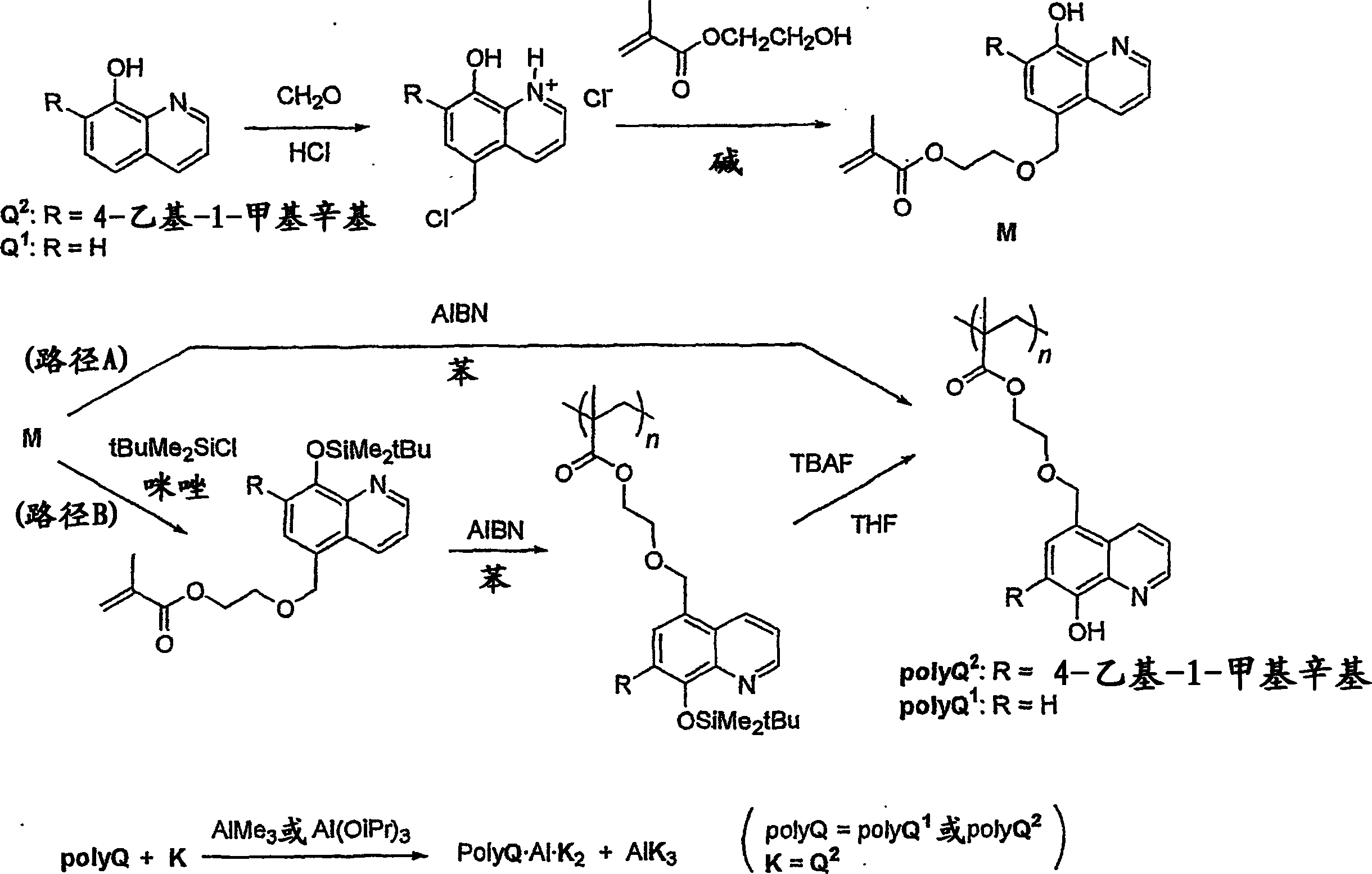
[0045] 8-hydroxyquinoline (Q 1 ) 26.2 g (0.201 mol), concentrated hydrochloric acid 36 mL, and 28 wt%-formalin 32 mL (0.3 mol), and hydrogen chloride gas dried at room temperature for 90 minutes was introduced into the obtained mixed solution.
[0046] After the reaction was over, the resulting precipitate was filtered and dried to obtain the chloromethylated product of an amorphous yellow solid (Q 1 -CH 2 Cl·HCl) 37.2 g (yield 80%). The melting point of the obtained product is 280° C. (decomposition), which is in good agreement with the literature value (J. Org. Chem. 26, p4078 (1961); 280° C. (decomposition).
[0047] Then, the chloromethylation product (Q 1 -CH 2 14.5 g (0.0629 mol) of Cl·HCl) and 60 mL (0.43 mol) of excess 2-hydroxyethyl methacrylate (HMA) were reacted at 60° C. for 2 days under an argon atmosphere.
[0048] After the reaction was completed and returned to room temperature, 150 mL of water was added, ...
Synthetic example 2
[0054] Synthesis example 2 (polyQ 2 Synthesis)
[0055] Charge 7-(4-ethyl-1-methyloctyl)-8-hydroxyquinoline (Q 2 ) 45.0g (0.15mol), concentrated hydrochloric acid 75mL, and 28wt%-formalin 25mL (0.23mol), and introduced hydrogen chloride gas dried at 65-75°C for 17 hours into the resulting mixed solution. The resulting reaction was a mixture of an aqueous layer and a dark yellowish-orange solid.
[0056] After completion of the reaction, extraction was performed four times with 100 mL of chloroform from which the stabilizer was removed, and the obtained extract was reprecipitated with 4 L of ether to obtain 23 g of an amorphous yellow solid product.
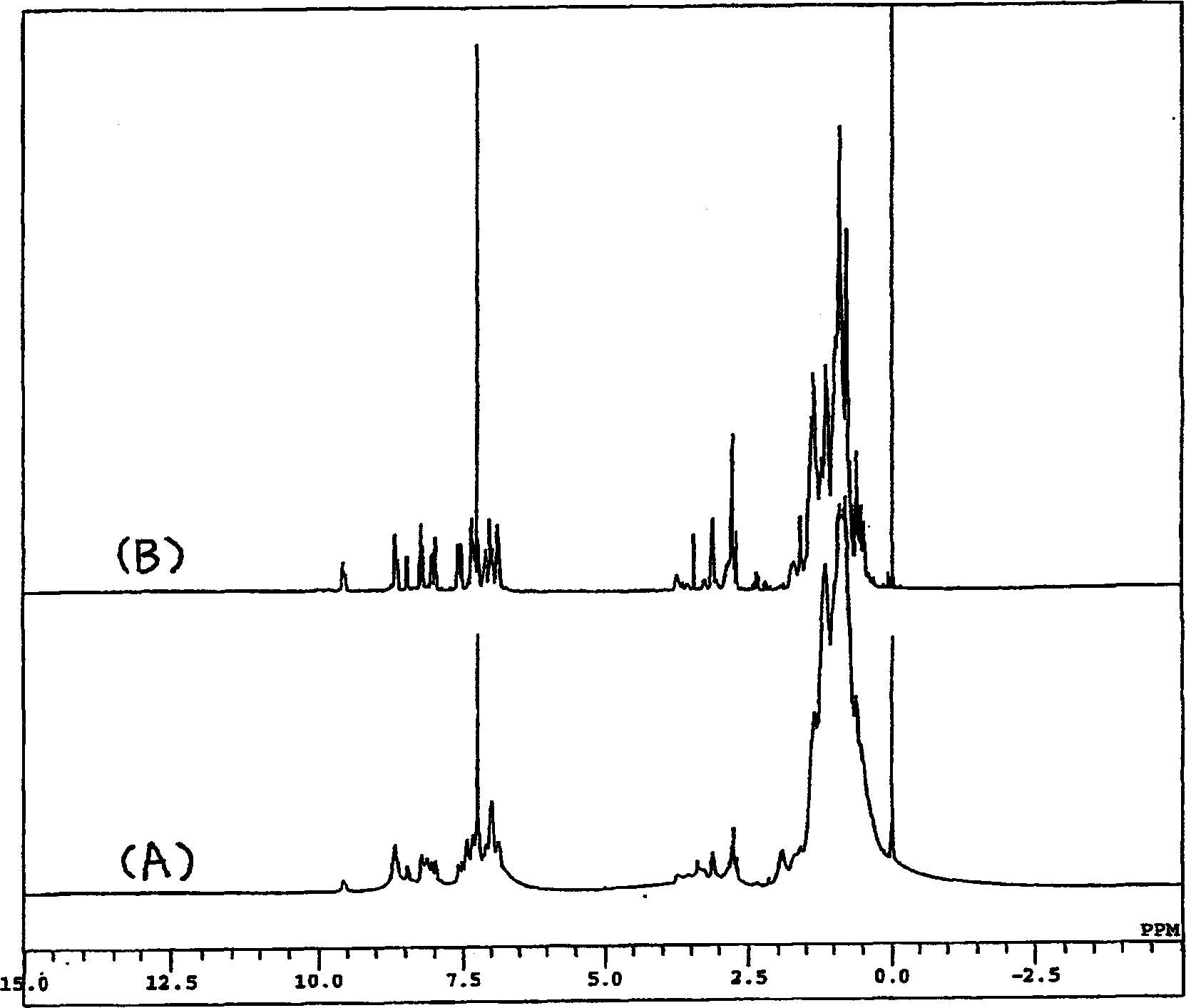
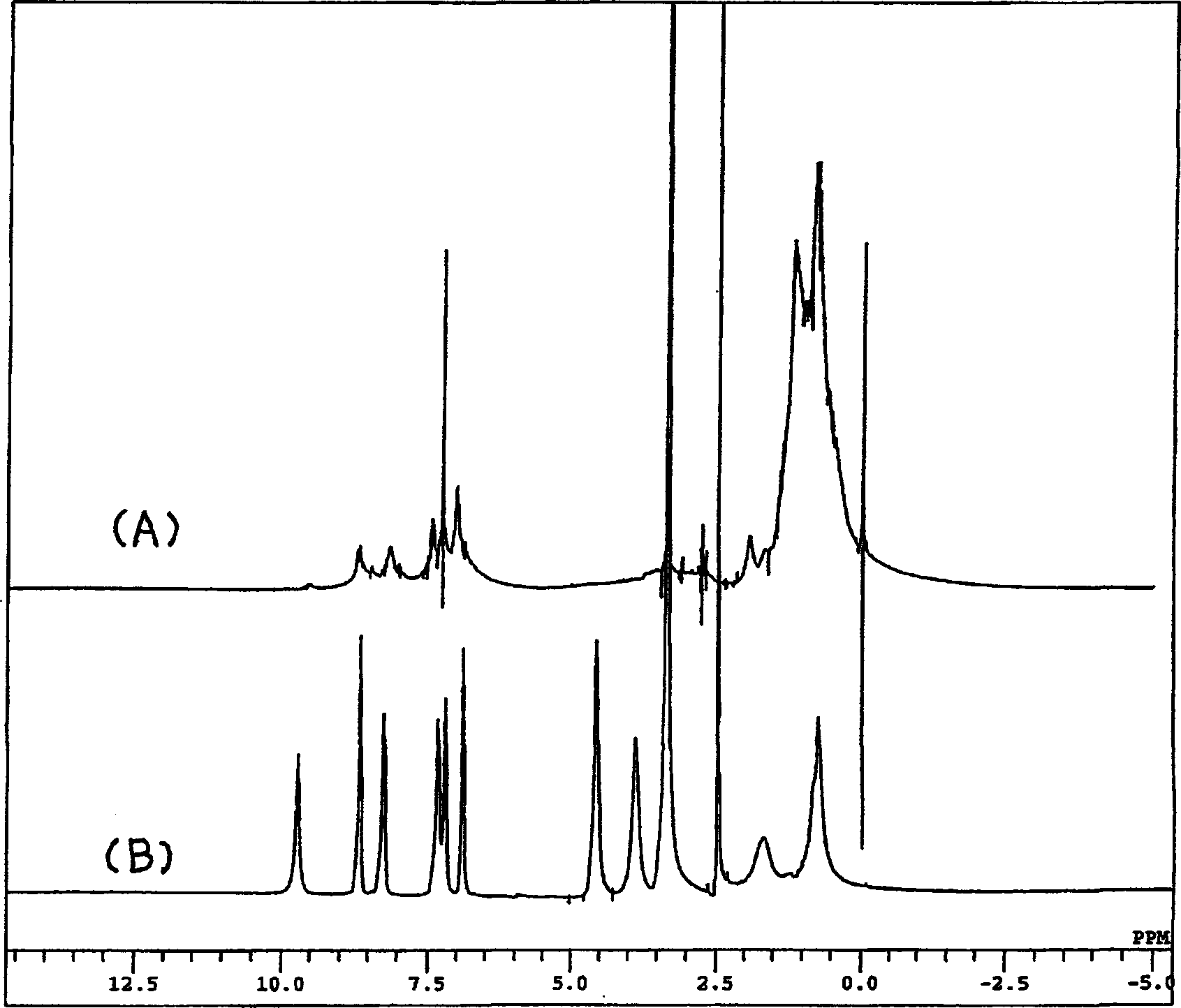
[0057] The resulting product is 1 H-NMR and 13 Each spectrum of C-NMR confirmed that the 5-position was chloromethylated (Q 2 -CH 2 Cl·HCl) (57% yield).
[0058] 1 H-NMR (400MHz; CDCl 3 )δ (ppm) = 9.14 (1H, d, J = 7.2Hz), 9.04 (1H, br.s), 7.99 (1H, br.s), 7.75 (1H, s), 8.06 (2H, s), 3.68 (1H, m), 1.64 (2H, br.d, J=5.6Hz)...
Synthetic example 3
[0069] Synthesis example 3 (polyQ 2 Synthesis)
[0070] The product obtained in the same manner as in Synthesis Example 2 above (Q2 -CH 2 2.13 g (4.82 mmol) of HMA) and 0.872 g (5.79 mmol) of tert-butyldimethylsilyl chloride were dissolved in dry DMF (5 mL), and imidazole was added to the resulting solution while stirring at room temperature. 0.82 g (12.0 mmol), and after stirring at room temperature for 10 hours, saturated sodium bicarbonate water was added to stop the reaction, followed by extraction with hexane. After drying the obtained organic layer, it was purified by silica gel column chromatography (hexane-ethyl acetate) to obtain a colorless oily product [Q 2 (OSi)-CH 2 -HMA] 2.68g (yield over 99%).
[0071] The resulting product [Q 2 (OSi)-CH 2 -HMA] 1 H-NMR data are as follows.
[0072] 1 H-NMR (400MHz; CDCl 3 )δ (ppm) = 8.76 (1H, dd, 2, 4Hz), 8.41 (1H, dd, 2, 9Hz), 7.35 (1H, s), 7.32 (1H, dd, 4, 9Hz), 6.08 (1H, br.s), 5.55 (1H, quintet, 1Hz), 4.91 (2H, s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com