Gen-seng saponin Rg2 preparation method, its pharmaceutical composition and uses in pharmacy
A technology of ginsenosides and lower alkanols, which is applied in the directions of drug combinations, steroids, and medical preparations containing active ingredients, etc. It is not suitable for large-scale production and other problems, and achieves the effect of good treatment of dementia, shortening sleep time, and regulating brain nerve cell apoptosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment Embodiment 1
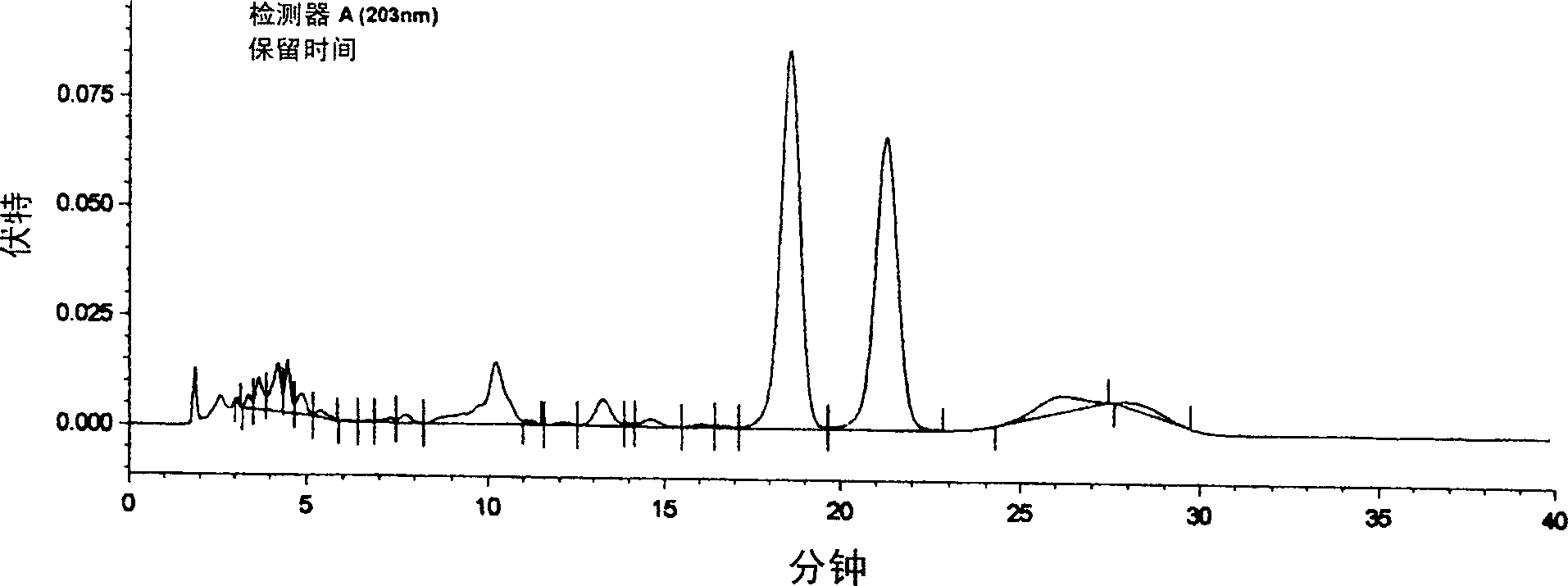
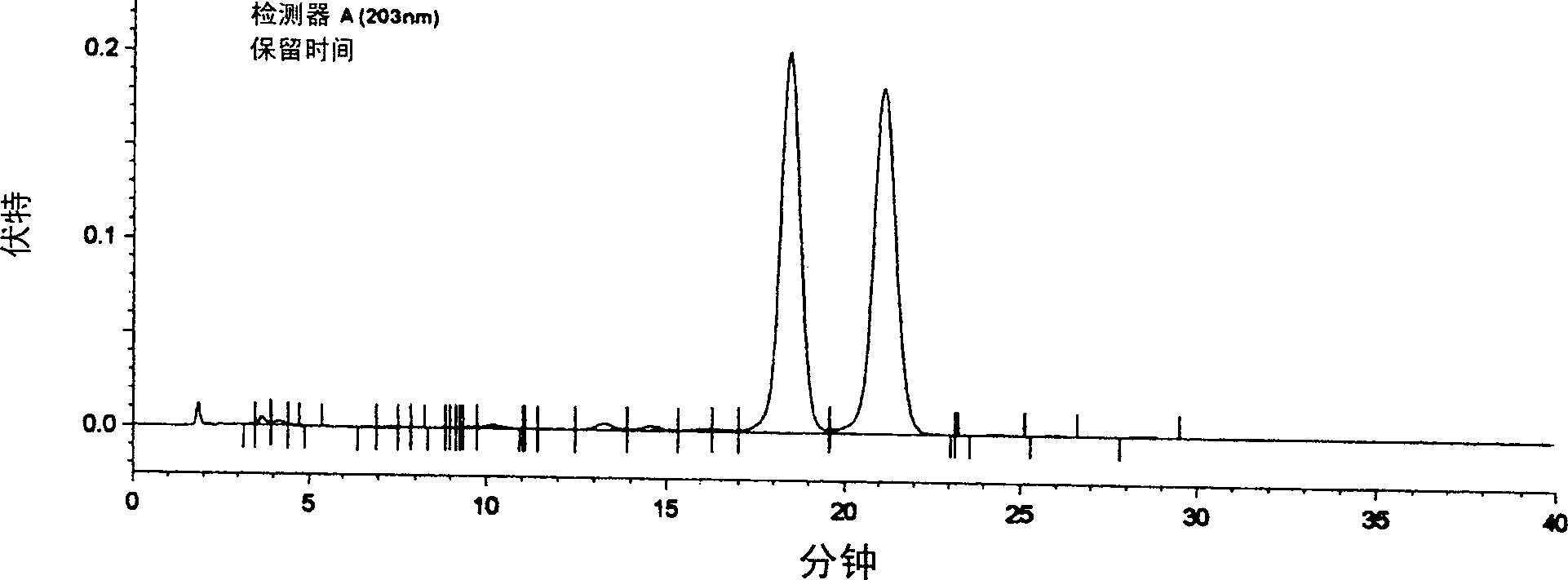
[0044] Experimental Example 1: Ginsenoside Rg 2 Extraction and separation method
[0045] Take 100kg of ginseng leaf meal, decoct three times in water for 3 hours each time, combine the decoctions, filter, concentrate the filtrate, add 3 times the amount of 95% ethanol to precipitate impurities, discard the sediment, decolorize with activated carbon, and recover ethanol. After the ethanol extract was dissolved in 10 times of water, ammonium sulfate (agricultural fertilizer) or sodium chloride (edible salt) was added to make the solution concentration reach saturation, and static precipitation was carried out for 8 hours. Take the precipitate, dissolve it in water again, carry out gradient (5%, 10%, 15%, 20%, 30% concentration) salting-out with sodium chloride or ammonium sulfate, respectively, and collect the precipitate respectively. Due to the different amount of solute in the solution, the gradient salting-out concentration is different. Therefore, under thin-layer chroma...
experiment Embodiment 2
[0059] Experimental Example 2: Ginsenoside Rg 2 anti-dementia effect
[0060] (1) Ginsenoside Rg 2 Injection of amyloid (Aβ 1-40 ) caused by memory impairment
[0061] The experiment used Alzheimer's dementia (AD) model. Take 70 rats, divide them into 7 groups, and anesthetize them with chloral hydrate. Except for the sham operation group, the other groups are injected with Aβ in the CA1 region of the hippocampus. 1-40 (4 μg) and ibotenic acid (1 μg) in a total of 1 μL. After the model was established, the test drugs were given the doses shown in Table 1.1 ip, and the model group was given the same volume of normal saline ip, once a day, for a total of 7 days. The test drug used is a single configuration C-20 (S) type ginsenoside Rg 2 , single configuration C-20 (R) type ginsenoside Rg 2 And Mixed C-20(SR) Ginsenoside Rg 2 (The following experiments are referred to as S, R, SR for short). On the 6th day of administration, a Y-type electric maze test was performed to o...
experiment Embodiment 3
[0094] Experimental Example 3: Antidepressant and Central Exciting Effects
[0095] (1) Effects on the contents of NE, DA, and 5-HT in rat brain tissue
[0096] Commonly used models of depression use drugs or stress stimulation to reduce the content of NE, DA, and 5-HT in brain tissue, causing central depression. In this experiment, cerebral ischemia-reperfusion method was used to reduce the content of NE, DA and 5-HT in brain tissue.
[0097] 56 rats were taken, divided into 7 groups, and anesthetized. Except for the sham operation group, each rat was introduced into the internal carotid artery with a suture (4-0 polyethylene line) from the left external carotid artery, and the middle cerebral artery (MCA) was blocked. ). The timing starts from blocking the blood flow, and after 0.5 hours, pull the tie line and reperfuse. Animals in each group received the test drugs ip at the dose shown in Table 4.1 on the operation day, postoperative day 1, day 2, day 3, and day 4, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com