Influenza virus vaccine
A type of influenza virus and vaccine technology, applied in the direction of virus antigen components, antibody medical components, fermentation, etc., can solve the problem of not being able to extend type B influenza virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Preparation of peptides
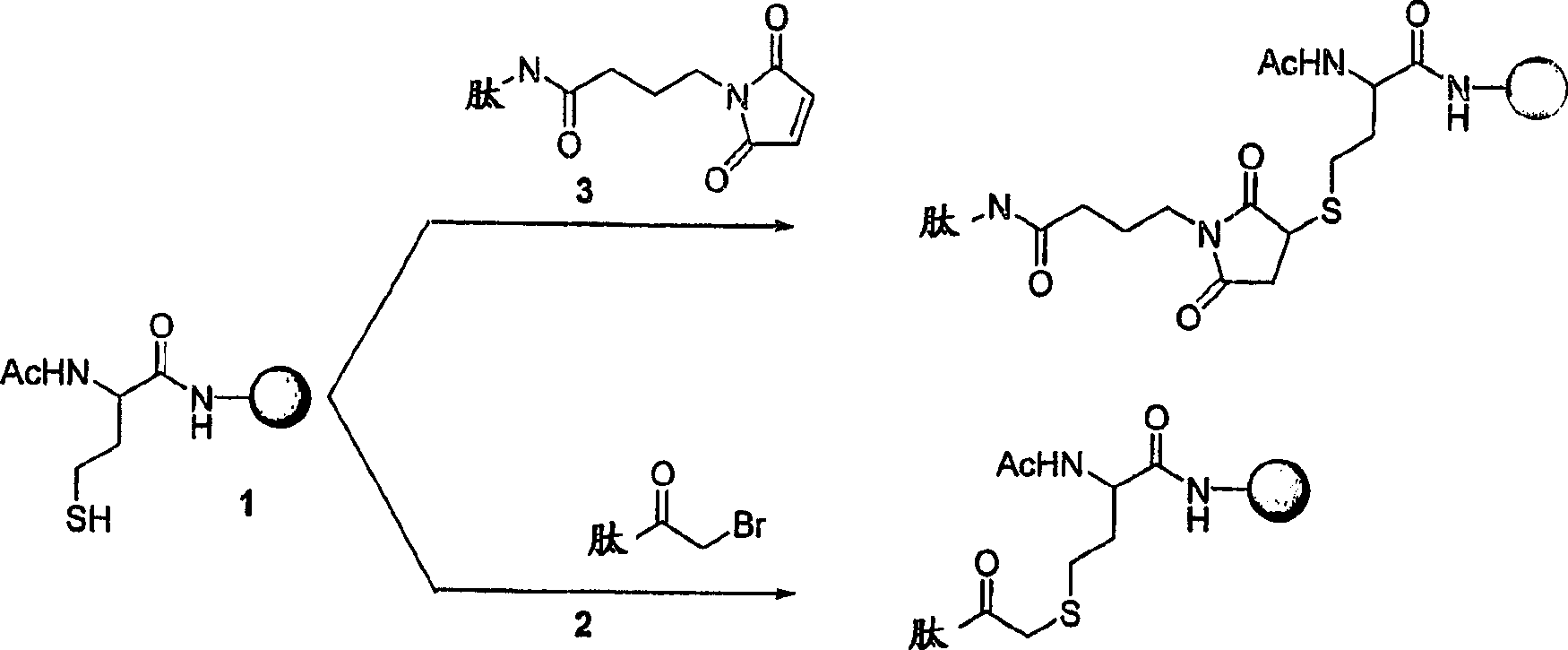
[0149] A synthetic peptide representing a portion of the M2 protein sequence and containing a C-terminal or N-terminal reactive bromoacetyl or maleimide group is prepared by a solid-phase chemical synthesis method commonly used in the art.
[0150] For example, C-terminal bromoacetylated M215-mer, CT-BrAcM2-15mer, Ac-Ser-Leu-Leu-Thr-Glu-Val-Glu-Thr-Pro-Ile-Arg-Asn-Glu-Trp -Gly-Aha-Lys(N ε -BrAc)-NH2 TFA salt (SEQ ID NO: 13), synthesized as a peptide bound to a protected resin on the APPLIEDBIOSYSTEMS 430A peptide synthesizer (APPLIED BIOSYSTEMS, CITY STATE). Starting with 0.5 mmoles of p-methylbenzylamine (MBHA) resin, this method uses a 4-fold excess (2 mmoles) of various N α -Boc protected amino acid. Side chain protection is Lys (Fmoc), Trp (formyl), Glu (OcHex), Arg (Tos), Thr (Bzl). The coupling is achieved by the activation of DCC and HOBT in methyl-2-pyrrolidone (NMP). Couple with acetic acid and introduce N-terminal acetyl group. Use 1:1TF...
Embodiment 2
[0191] Preparation of thiolated outer membrane protein complex (OMPC) of Neisseria meningitidis
[0192] The OMPC is obtained using techniques known in the art and described in US Patent No. 5,494,808. Using the general method described by Marburg et al. 1986, the thiolation with N-acetyl homocysteine lactone was prepared by using aseptic technique. For NT-BrAcM2-15 and CT-BrAcM2-15, the thiolated OMPC was finally resuspended in N 2 Saturated 25mM borate, 0.15M NaCl, 2mM EDTA, pH 8.5; for reaction with NT-MalM2-15 and CT-MalM2-15, resuspend in 20mM HEPES, 0.15M NaCl, 2mM EDTA, pH 7.3. By appropriately diluting the thiolated OMPC to N 2 The thiol content was measured in saturated 0.1M sodium phosphate, 0.1M NaCl, 2mM EDTA, pH 7 buffer. Use N 2 A 50 mM DTNB stock solution saturated with 0.1 M sodium phosphate, 0.1 M NaCl, 2 mM EDTA, pH 7 buffer will add DTNB to a final concentration of 5 mM. After incubating for 15 minutes at room temperature, after subtracting the appropriate DT...
Embodiment 3
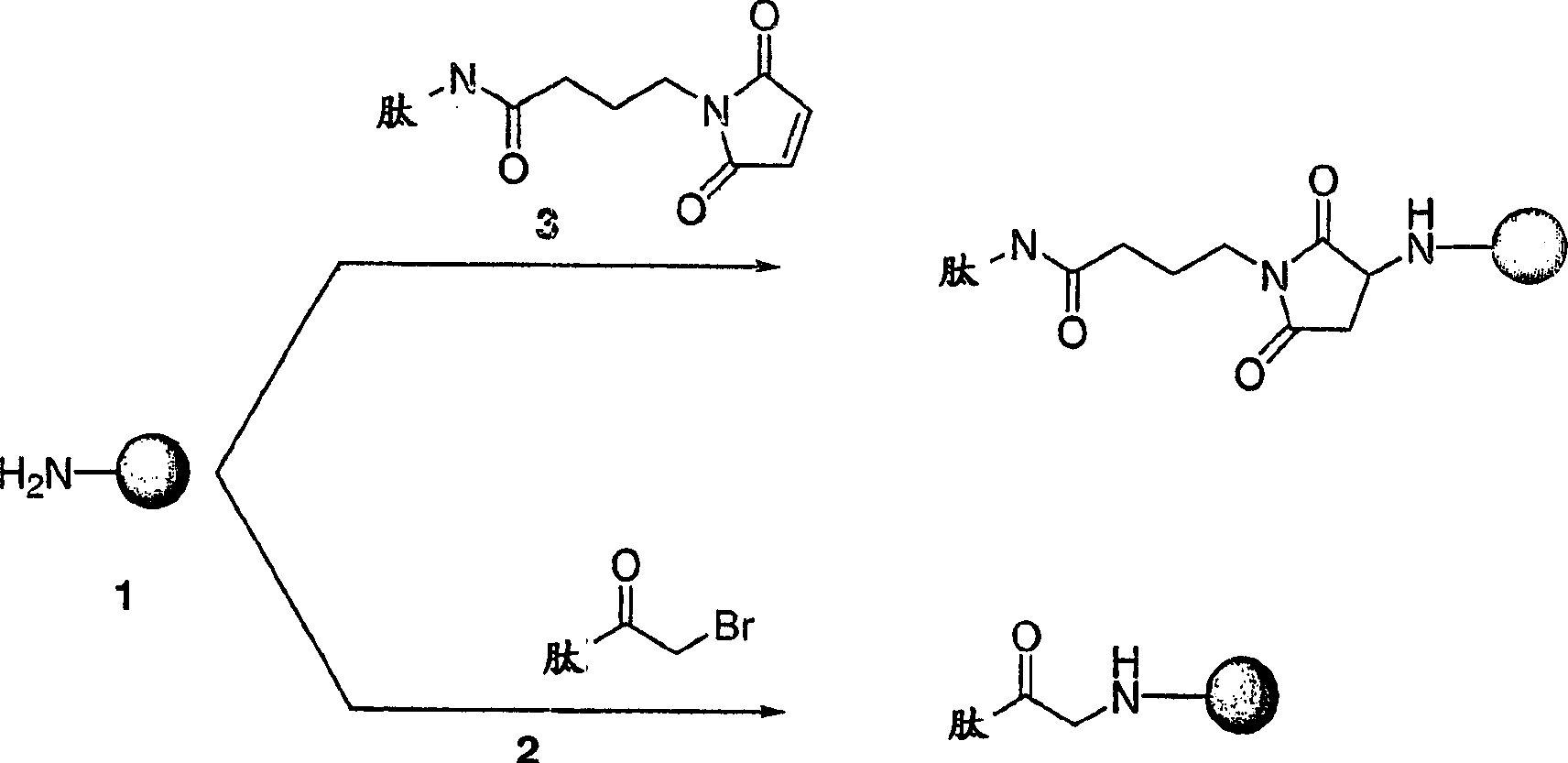
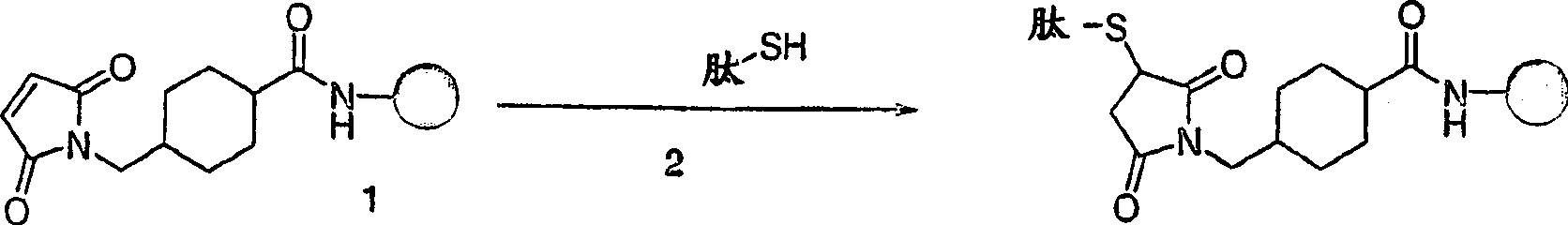
[0197] Preparation of maleimidized or alkyl halide-activated OMPC
[0198] All operations are performed aseptically. By adding a suitable volume of sterile 0.5M NaHCO 3 At NaHCO 3 Prepare a 50mM sterile OMPC aqueous solution (5.5mg / mL) at pH 8.5±0.1. Add 4-(N-maleimidomethyl)cyclohexane-1-carboxylic acid sulfosuccinimide ester (sSMCC) or (4-iodoacetyl)amino group dropwise to the buffered OMPC with gentle stirring Sulfosuccinimide benzoate (sSIAB) (10mM ice-cold H 2 Stock solution in O; chemicals purchased from PIERCE CHEMICAL CO., ROCKFORD, IL), giving a final concentration of 2.5 mM sSIAB or sSMCC and an OMPC concentration of approximately 3.8 mg / mL. N-hydroxysulfosuccinimide bromoacetate can also be used. The reaction was aged for 1 hour at 4°C and protected from light. After 1 hour, the reaction mixture was adjusted to pH 7.3 with sterile 1M sodium phosphate, and 300K molecular weight cut-off (MWCO) DISPODIALYZER (SPECTRUM INDUSTRIES, INC., RANCODOMINGUEZ, CA) with sterile 6.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com