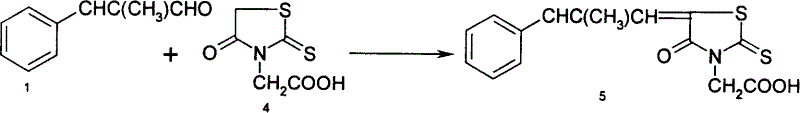Method for preparing thiazolidine acetate coprising phenyl propylidene
A technology of thiazolidine acetic acid and phenylpropenylidene is applied in the preparation field containing phenylpropenylidene thiazolidine acetic acid, and can solve the problems of low reaction yield, complicated post-processing, difficulty in recovery and application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
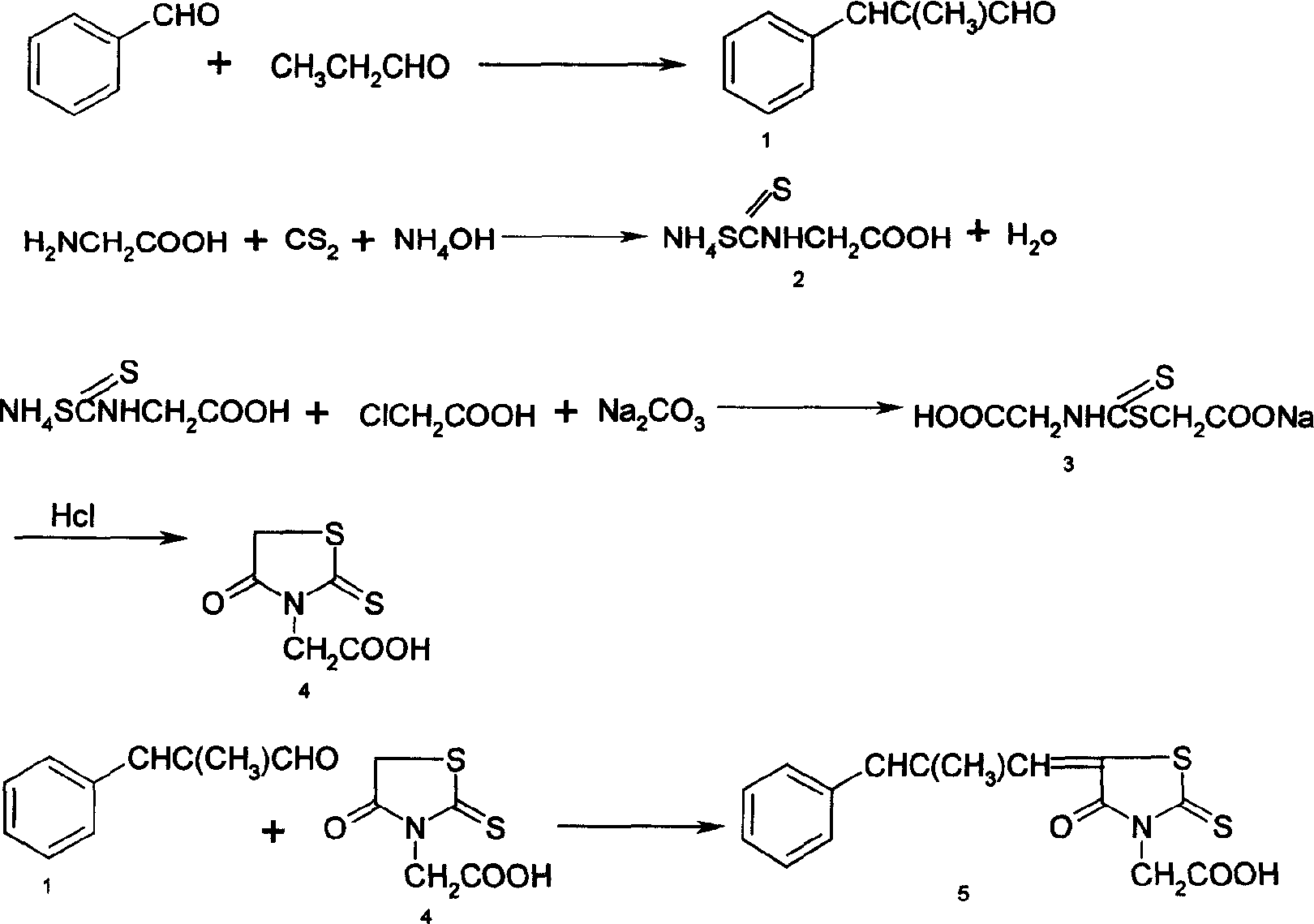
[0011] Synthesis of α-Methylcinnamaldehyde 1
[0012] Dissolve 26.1g NaOH in 285ml water, stir to dissolve, add 135.3g benzaldehyde, add 37g propionaldehyde dropwise at below 50°C, after the addition is complete, continue to stir and react for 2 hours. After the reaction, separate the oil layer, distill under reduced pressure, and recover For benzaldehyde, the fraction with bp of 120-124°C / 9-13mmHg is collected, and the yield is about 80% for propionaldehyde.
[0013] Rhodanine acetic acid is the synthesis of compound 4:
[0014] a. Dissolve 40g of glycine in a mixture of 33ml of water and 100ml of ammonia water, add 35ml of carbon disulfide dropwise at a temperature below 10°C, react at room temperature for 2 hours, add 150ml of ethanol to crystallize, filter, rinse with ethanol and dry to obtain the disulfide compound 2 .
[0015] b. Add 57.5g of chloroacetic acid, 32.5g of sodium carbonate and 144ml of water into a 500ml three-necked flask, add all of the above-mentioned ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com