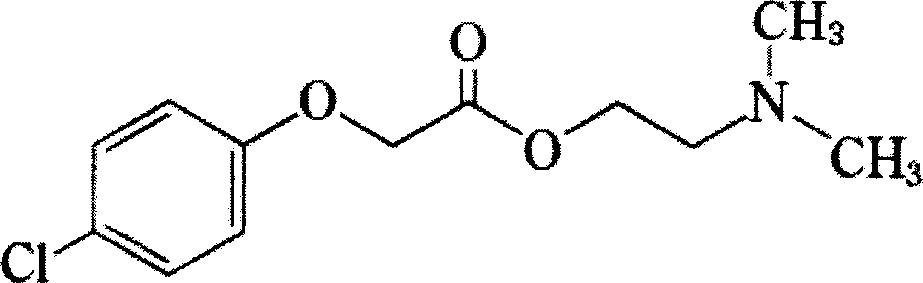
Liquid medicine containing lucidril and preparing method
A liquid medicine and medicine technology, applied in the field of medicine, can solve the problems of high production cost, long preparation period of powder injection, high energy consumption, and achieve the effects of convenient taking, increasing the chance of drug contamination, and increasing operation procedures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1: the liquid medicament containing chlorphenal
[0077] Prescription (according to 1000ml):
[0078] Meclofenoxate Hydrochloride 50g
[0079] Absolute ethanol 200ml
[0080] 1,2-Propanediol was added to make up to 1000ml.
[0081] Preparation:
[0082] Under the condition of meeting the GMP requirements for pharmaceutical manufacturing, take the prescribed amount of meclofenoxate hydrochloride, add 200ml of absolute ethanol, stir to dissolve, then add 1,2-propanediol to 1000ml, and stir evenly.
[0083] Filter with a 0.45 μm microporous membrane, take 500ml of the filtrate and fill it in ampoules, each with 5ml, fill with nitrogen, seal, and sterilize at 100°C for 15min to make an injection containing meclofenoxate hydrochloride.
[0084] After filtering with a 0.45 μm microporous membrane, the remaining filtrate is filled in a medicinal oral liquid glass bottle, 5 ml per bottle, filled with nitrogen, and sealed to make an ...
Embodiment 2
[0085] Embodiment 2: the liquid medicament containing chlorphenal
[0086] Prescription (according to 1000ml):
[0087] Meclofenoxate Hydrochloride 50g
[0088] Absolute ethanol 300ml
[0089] Add polyethylene glycol-300 to 1000ml.
[0090] Preparation:
[0091] Under the condition of meeting the GMP requirements for pharmaceutical manufacturing, take the aseptic, pyrogen-free meclofenoxate hydrochloride of the prescribed amount, add 300ml of absolute ethanol, stir to dissolve, then add polyethylene glycol to 1000ml, and stir evenly.
[0092] Filter and sterilize with a 0.22 μm microporous membrane, get 500ml of the filtrate and fill it in ampoules, each with 5ml, fill with nitrogen, and seal to make an injection containing meclofenoxate hydrochloride.
[0093] After filtering with a 0.22 μm microporous membrane, the remaining filtrate is filled in a medicinal oral liquid glass bottle, 5 ml per bottle, filled with nitrogen, and sealed to make ...
Embodiment 3
[0094] Embodiment 3: the liquid medicament that contains perchlorate
[0095]Prescription (according to 1000ml):
[0096] Meclofenoxate Hydrochloride 50g
[0097] Benzyl alcohol 10g
[0098] Absolute ethanol 400ml
[0099] Glycerin was added to 1000ml.
[0100] Preparation:
[0101] Under the condition of meeting the GMP requirements for pharmaceutical manufacturing, take the prescribed amount of meclofenoxate hydrochloride and benzyl alcohol, add 400ml of absolute ethanol, stir and dissolve, then add glycerin to 1000ml, and stir evenly.
[0102] Filter with a 0.45 μm microporous membrane, take 500ml of the filtrate and fill it in ampoules, each with 5ml, fill with nitrogen, seal, and sterilize at 100°C for 15min to make an injection containing meclofenoxate hydrochloride.
[0103] After filtering with a 0.45 μm microporous membrane, the remaining filtrate is filled in a medicinal oral liquid plastic bottle, 5 ml per bottle, fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com