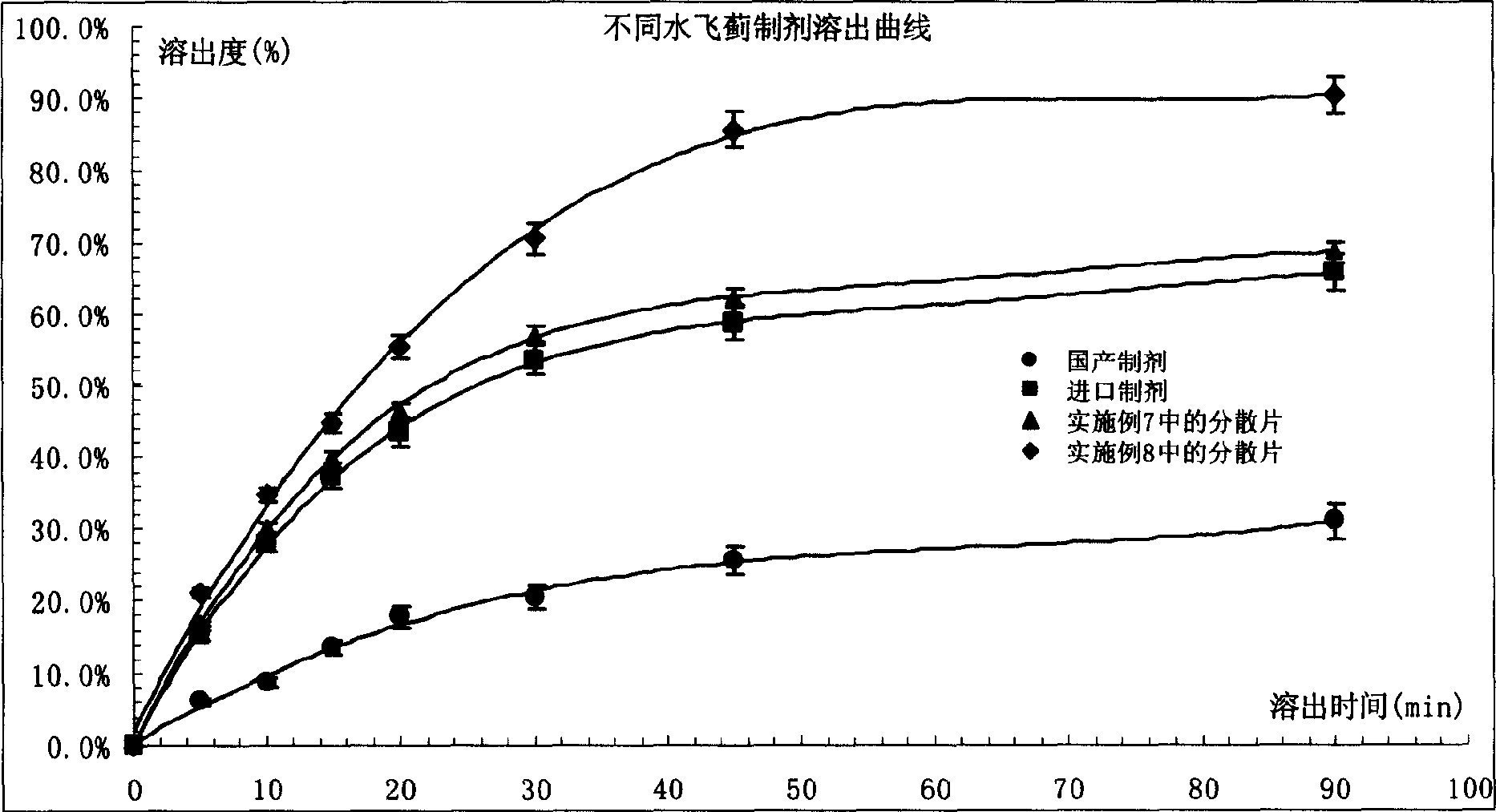
Development of micro particle silybum marianum preparation
A technology of silymarin and micronization, which is applied in pill delivery, digestive system, antiviral agent, etc., can solve the problems of cost increase, low bioavailability of silymarin, environmental pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1. Preparation of silymarin-phospholipid solid dispersion
[0045] Weigh 100g of medicinal-grade phospholipids, add 600ml of absolute ethanol, stir thoroughly to completely dissolve the phospholipids in the solvent; then add 100g of silymarin, sonicate and moderately heat to completely dissolve the silymarin; filter while hot; take the filtrate and evaporate under reduced pressure at 60°C Ethanol; vacuum drying at 60°C to remove residual solvent; pulverization to obtain a silymarin-phospholipid solid dispersion.
Embodiment 2
[0046] Example 2. Preparation of silymarin-poloxamer solid dispersion
[0047] Weigh 150g of poloxamer 188 and heat it on a 60°C water bath. After all the poloxamer is melted, weigh 10g of silymarin and add it to the molten poloxamer. Stir thoroughly. After all the poloxamers are mixed, -25 Cool quickly at ℃, place it in a desiccator for a few days to fully dry and pulverize to obtain a silymarin-poloxamer solid dispersion.
Embodiment 3
[0048] Example 3. Preparation of silymarin-PEG 6000 solid dispersion
[0049] Weigh 400g of PEG 6000, place it on a water bath, and heat and melt at 70°C. After all the PEG is melted, add 100g of silymarin, stir and mix well, freeze and rapidly cool at -20°C, place it in a desiccator, and pulverize to obtain silymarin. -PEG solid dispersion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com