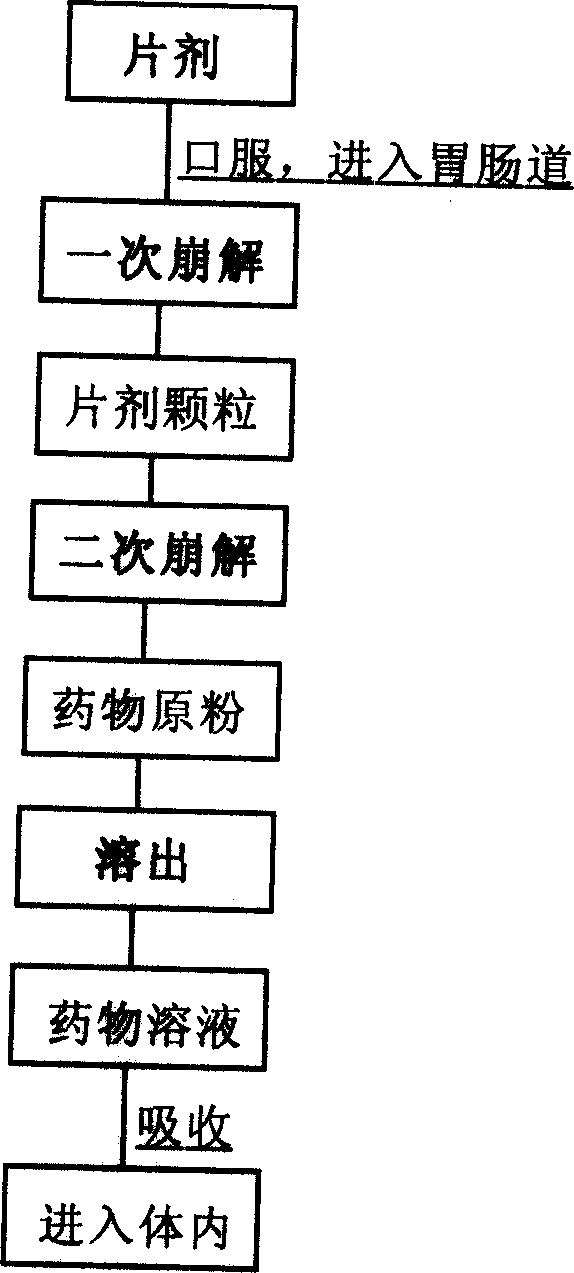
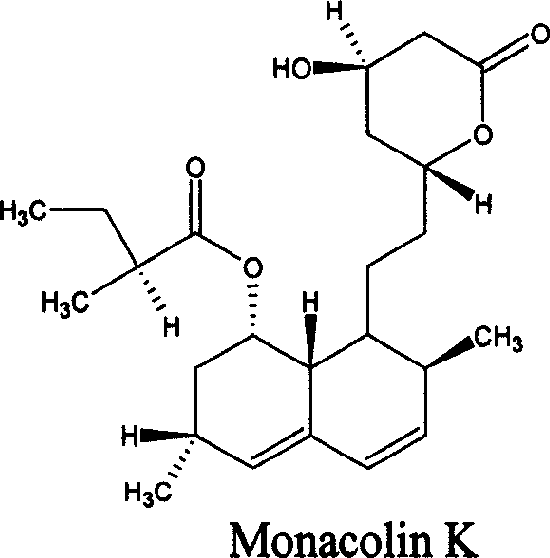
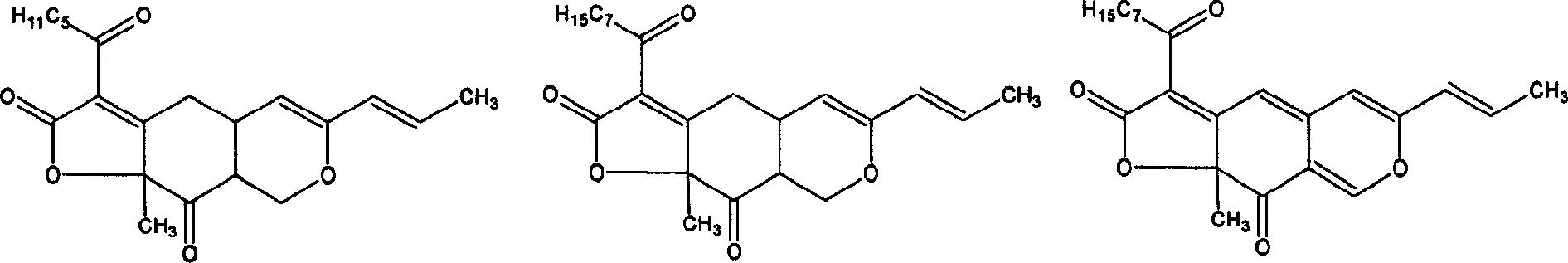
Preparation and application of red rice dispersible tablet
A technology of dispersible tablets and red yeast rice, which is applied in the field of medicine, can solve the problems of smaller particles of the main drug, poor dissolution, and increased surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Prescription of Red Yeast Dispersible Tablets:
[0074] Red yeast rice 300g
[0075] CMS-Na 25g
[0076] Microcrystalline Cellulose 100g
[0077] L-HPC 25g
[0078] Crosslinked CMC-Na 25g
[0079] Micronized silica gel 6g
[0080] Magnesium Stearate 4.5g
[0081] 80% ethanol appropriate amount
[0082] Made into 1000 pieces
[0083] Preparation method: Weigh red yeast rice according to the above prescription, put it in a particle ball mill and grind it for 2 hours, take it out and pass it through a 120-mesh sieve, and weigh microcrystalline cellulose, L-HPC, cross-linked CMC-Na according to the prescription amount, 2 / 3 Prescribed amount of CMS-Na, mixed evenly; soft material made of 95% ethanol, granulated with 24 mesh, dried at 60°C, granulated, added the remaining amount of CMS-Na, micron...
Embodiment 2
[0086] Prescription of Red Yeast Dispersible Tablets:
[0087] Red Yeast Extract 50g
[0088] Low Substitution-Hydroxypropyl Cellulose 20g
[0089] Microcrystalline Cellulose 100g
[0090] Lactose 100g
[0091] 5% PVP K30 Solution 100g
[0093] Made into 1000 pieces
[0094] Preparation method: Take red yeast rice and add 5 times the volume of ethanol, ultrasonically extract, 30min each time, extract twice, combine the extracts, recover under reduced pressure, and obtain red yeast rice extract; weigh the red yeast rice extract and lactose according to the above preparation prescription , placed in an airflow mill and crushed, passed through a 200-mesh sieve, added microcrystalline cellulose and low-substituted-hydroxypropyl cellulose, mixed evenly, and mixed with 5% PVP K30 Soft material made from ethano...
Embodiment 3
[0097] Prescription of Red Yeast Dispersible Tablets:
[0098] Red Yeast Extract 200g
[0099] Low Substitution-Hydroxypropyl Cellulose 20g
[0100] Microcrystalline Cellulose 50g
[0101] Sodium Lauryl Sulfate 4g
[0102] Lactose 50g
[0103] 5% PVP K30 Solution 100g
[0105] Made into 1000 pieces
[0106] Preparation method: take red yeast rice and add 5 times the volume of 70% acetone solution, heat reflux extraction, 60 minutes each time, extract twice, combine the extracts, recover under reduced pressure, and obtain red yeast rice extract; weigh red yeast rice according to the above prescription The extract, lactose and sodium lauryl sulfate were pulverized in a jet mill, passed through a 200-mesh sieve, added microcrystalline cellulose and 1 / 2 prescription amount of low-substituted-hydroxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com