Heterochain polymer chiral stationary phase and process for preparing same
A chiral stationary phase and polymer technology, which is applied in the field of heterochain polymer chiral stationary phase and its preparation, can solve the problems of high cost, cumbersome operation, and high price of chiral chromatographic columns, and simplify the preparation steps , easy preparation, and high practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] 2. Preparation method of heterochain polymer chiral stationary phase
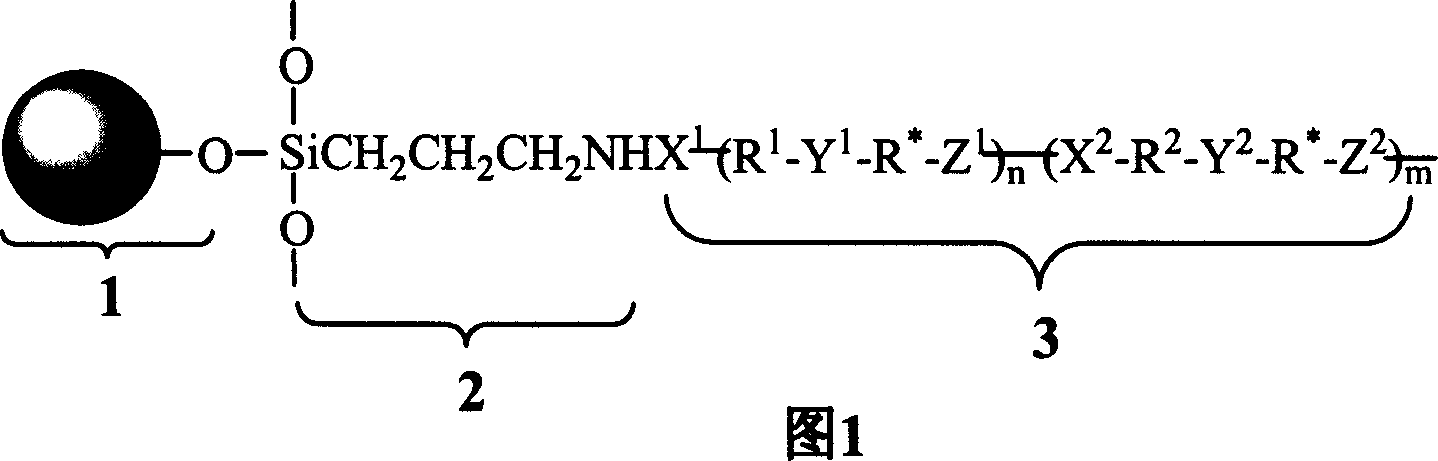
[0037] It is formed by connecting a carrier and a chiral polymer through an amide bond or a urea bond, and the carrier is 3-aminopropyl silica gel.
[0038] The chiral polymer is one of polyamide, polyester, polyurethane, poly(ester-amide), poly(ester-urethane), or poly(amide-urea). Chiral polymers are polymerized by monomers, that is, through chiral diamines or chiral diols or chiral diphenols or chiral alcohol amines, and one or both of excess diacid chlorides and diisocyanates It is polymerized from species (that is, including diacyl chloride and diisocyanate), and the end segment has an acid chloride or isocyanate active group that can react with the functional group on the silica gel. One or both of diacyl chloride and diisocyanate are calculated based on the moles of monomers charged during the reaction, and the excess range is 1 to 20%.
[0039] Among the above-mentioned monomers used, at le...
Embodiment 1
[0040] Example 1. Preparation of polyamide chiral stationary phase
[0041] Add 5.22 grams of terephthaloyl dichloride and 5.22 grams of D-1,2-diphenylethylenediamine into 50 milliliters of dimethylformamide (DMF), stir, then add 10 milliliters of triethylamine, at 30-100 Reaction at ℃ for 5-15 hours. DMF was evaporated under reduced pressure, and the residue was washed with n-hexane. The washed solid was transferred to a three-neck flask, 30 ml of DMF, 3 ml of triethylamine and 4 g of 3-aminopropyl silica gel were added, and the reaction was stirred at 20-70° C. for 8 hours. Filter, extract the solid with acetone, and dry to constant weight. The measured elemental analysis results of the chiral stationary phase are: C: 18.31%; H: 2.647%; N: 3.632%. Infrared spectral data (cm -1 , KBr): 3423 (-NH-CO-), 1691 (-NH-CO-), 1530 (-NH-CO-).
Embodiment 2
[0042] Example 2. Preparation of Polyurethane Chiral Stationary Phase
[0043] Add 3.38 g of p-phenyl diisocyanate and 4.65 g of diisopropyl tartrate into 40 ml of pyridine, and react at 30-100° C. for 5-15 hours. The reaction mixture was dropped into toluene to obtain a solid. The solid was redissolved with pyridine and precipitated three times with toluene. The precipitated polymer was dissolved in pyridine, 4 g of 3-aminopropyl silica gel was added, and the reaction was stirred at 20-70° C. for 12 hours. After filtration, the solid was extracted with acetone and dried to constant weight. The measured elemental analysis results of the chiral stationary phase are: C: 20.81%; H: 2.932%; N: 3.809%. Infrared spectral data (cm -1 , KBr): 3410 (-NH-CO-), 1721 (-CO 2 -), 1642 (-NH-CO-).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com