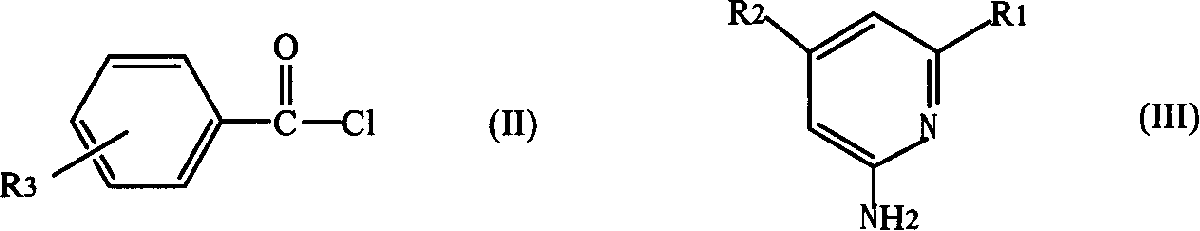Olefine oligomerization catalyst, and its preparation method and use
A catalyst and olefin technology, applied in the field of olefin oligomerization, can solve the problem of low oligomerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
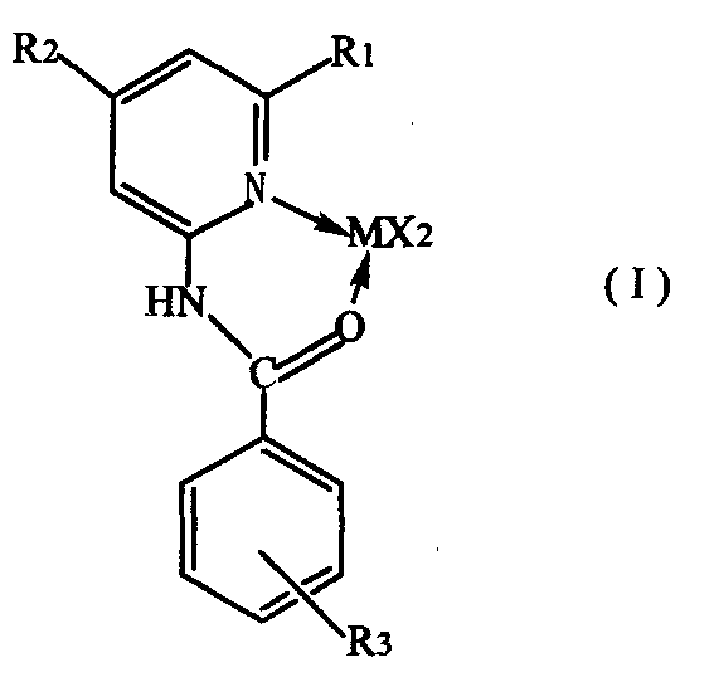
[0014] The preparation method of catalyst provided by the invention comprises the steps:
[0015] (1) benzoyl chloride derivatives shown in formula (II) are dissolved in tetrahydrofuran to make a solution, 2-aminopyridine derivatives shown in formula (III) are dissolved in pyridine to make a solution, and then described tetrahydrofuran The solution is added to the pyridine solution, and the compound of formula (II) and formula (III) is fully reacted at 20-100°C in a molar ratio of 1-1.1:1, then washed with water, and the solvent is removed to obtain N-pyridylbenzamides Ligand compound, R in the formula (II) 3 selected from hydrogen, nitro or -CF 3 , R in formula (III) 1 , R 2 respectively selected from hydrogen or C 1 ~C 6 the alkyl group;
[0016]
[0017] (2) In tetrahydrofuran medium, make N-pyridylbenzamide ligand and MX 2 or MX 2 DME is reacted at 20-100°C at a molar ratio of 1-1.2:1, and the MX 2 M is selected from Ni or Pd, X is selected from halogen, DME is...
example 1
[0027] Preparation of catalyst {N-[2-(6-methylpyridyl)]benzamide}nickel dibromide of the present invention.
[0028] (1) Preparation of ligand N-[2-(6-methylpyridyl)]benzamide
[0029]1.41 g of benzoyl chloride (10 mmol) was dissolved in tetrahydrofuran to form a solution of 10 ml, and this solution was added dropwise to 1.08 g of 2-amino-6-methylpyridine (10 mmol) in 10 ml at 20°C. ml of pyridine solution. React at 25°C for 12 hours, add 400 ml of deionized water to shake and wash to terminate the reaction, filter the washed material, and dry the filtered solid for 4 hours under reduced pressure to obtain 2.05 g of white solid, which is ligand a: N- [2-(6-Methylpyridyl)]benzamide, yield 99% by mass. The analysis results of the ligand are as follows:
[0030] FT-IR (KBr disc, cm -1 ): 3192(m), 1677(s), 1577(s), 1459(s), 1304(s), 1129(m), 790(m), 719(m).
[0031] 1 H NMR (300MHz, CDCl 3 / ppm): 2.47 (s, 3H, CH 3 ), 6.92-8.22 (m, 8H, ArH), 8.62 (s, 1H, NH).
[0032] Elem...
example 2
[0040] Prepare ligand b according to example 1 (1) step method: N-[2-(4,6-lutidine base)] benzamide, the difference is to use 2-amino-4,6-lutidine The reaction was carried out instead of 2-amino-6-picoline to obtain 1.43 g of a white solid, yield 63% by mass. The analysis results of the ligand are as follows:
[0041] FT-IR (KBr disc, cm -1 ): 3183(w), 1676(s), 1614(m), 1567(s), 1421(s), 1282(s), 846(w), 706(s).
[0042] 1 H NMR (300MHz, CDCl 3 / ppm): 2.37 (s, 3H, CH 3 ), 2.43 (s, 3H, CH 3 ), 6.78-7.94 (m, 7H, ArH), 8.05 (s, 1H, NH).
[0043] Elemental analysis, measured (calculated) value, mass %: C, 74.43 (74.31); H, 6.24 (6.24); N, 12.17 (12.38).
[0044] Prepare catalyst by the method for example 1 (3) step, difference is to react with the ligand b of 1.1 millimoles, obtains orange-red powder 0.3 gram, is catalyst G: {N-[2-(4,6-dimethyl Base pyridyl)] benzamide} nickel dibromide, yield 68% by mass. The analysis results are as follows:
[0045] FT-IR (KBr disc, cm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com