Freeze dried powder injection of hirudin and its preparation method
A technology for freeze-dried powder injection and hirudin, which is applied in the field of hirudin freeze-dried powder for injection and its preparation, can solve the problems of reduced inhibition constant, high environmental requirements, low purity of hirudin, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
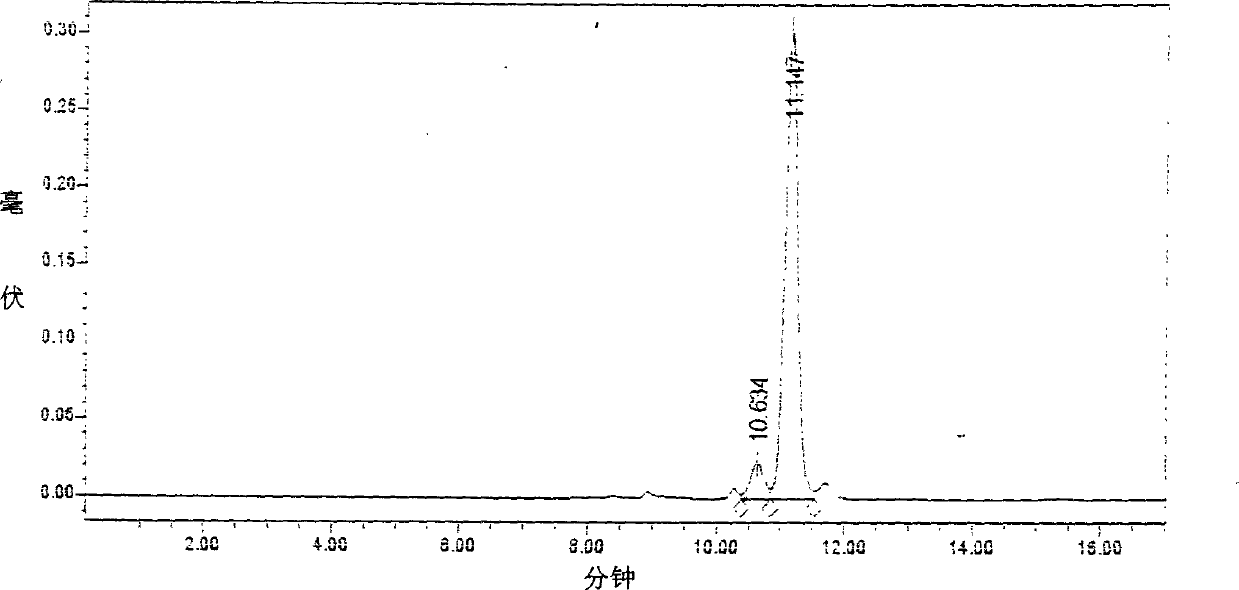
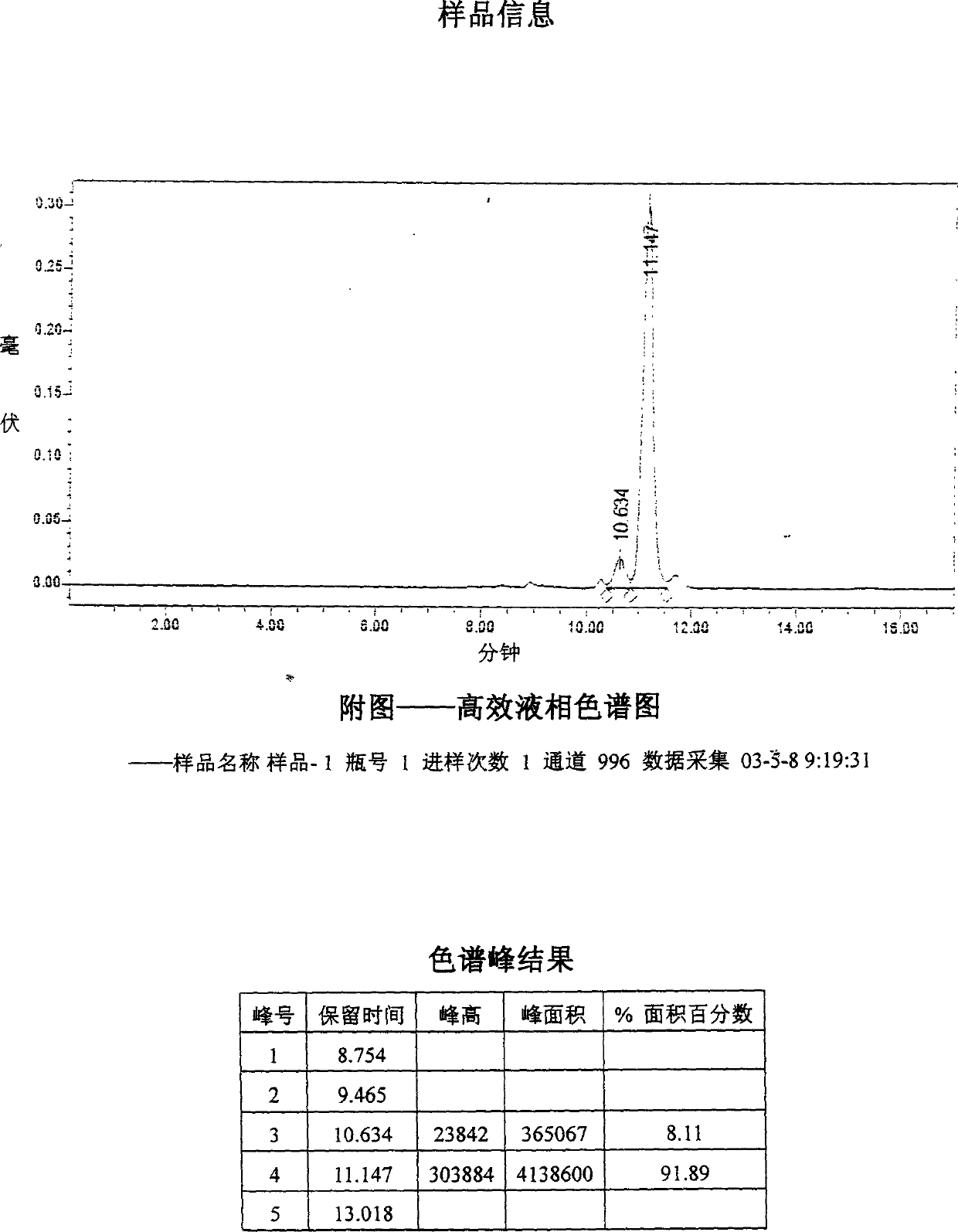
Image
Examples
Embodiment 1
[0027] Detection of specific activity of hirudin
[0028] 1. Experimental principle: The detection method of natural hirudin activity has not yet been published. According to the detection method of thrombin activity (Appendix 1 of the 2000 edition of the Chinese Biological Products Regulations), the ratio of hirudin and thrombin is 1:1. According to the principle of consuming one thrombin unit, which is equivalent to one anticoagulant unit (ATU), the potency of the anticoagulant unit of hirudin is detected by the test tube method (serial dilution method).
[0029] 2. Experimental materials: normal saline solution of 5g / L fibrinogen, normal saline for injection (provided by the laboratory of Beijing Qianluchun Technology Co., Ltd.)
[0030] Freeze-dried thrombin (purchased by Anhui Kuwahara Institute of Biotechnology, the content of each bottle is 500IU, diluted with normal saline to contain 0.5IU of thrombin per ml)
[0031] 3. Sample to be tested: the hirudin sample is the ...
Embodiment 2
[0039] The effect on thrombus formation in rats was determined by thrombus method.
[0040] 1. Experimental drug: normal saline (blank group) was provided by the laboratory of Beijing Qianluchun Technology Co., Ltd.
[0041] Leech Injection (Provided by Jilin Provincial Institute of Materia Medica)
[0042] Hirudin freeze-dried powder injection of the present invention (provided by the laboratory of Beijing Ganluchun Science and Technology Co., Ltd.)
[0043] 2. Experimental animals: 30 rats weighing 300-400 grams, male or female. Divide into three groups, 10 in each group.
[0044] 3. Experimental method: Rats were anesthetized (sodium pentobarbital 30-40mg / kg, ip), fixed in the supine position, the trachea was separated, a plastic cannula was inserted, and the right common carotid artery and left external jugular vein were separated. A 6 cm long silk thread was placed in the middle of the polyethylene tube, and the polyethylene tube was filled with...
Embodiment 3
[0049] Effect on ADP-induced thrombotic death in mice
[0050] Medicine: Freeze-dried powder injection of the present invention (provided by the laboratory of Beijing Ganluchun Technology Co., Ltd.)
[0051] Commercially available Ginkgo Damo injection (produced by Nanjing Jinling Pharmaceutical Co., Ltd., purchased from Beijing Baitasi Pharmaceutical Wholesale Company)
[0052] Normal saline (provided by the laboratory of Beijing Qianluchun Technology Co., Ltd.)
[0053] Method: Take 60 healthy mice, half male and half male, and randomly divide them into 3 groups: normal saline group, the freeze-dried powder injection group of the present invention, and the commercially available Ginkgo Damo injection group. Daily tail vein administration, continuous 7d, at the last time 1 hour after administration, ADP48.0mL / kg was injected into the tail vein, and the animal death was recorded. See Table 2:
[0054] group
Total / only
Number of deaths / only
d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com