Therapeutic compositions
A compound, hydroxybutyric acid technology, used in drug combinations, food ingredients containing organic compounds, food science, etc., can solve problems such as hypertriglyceridemia, increased vascular disease, pancreatic disease, and reduced leucine oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
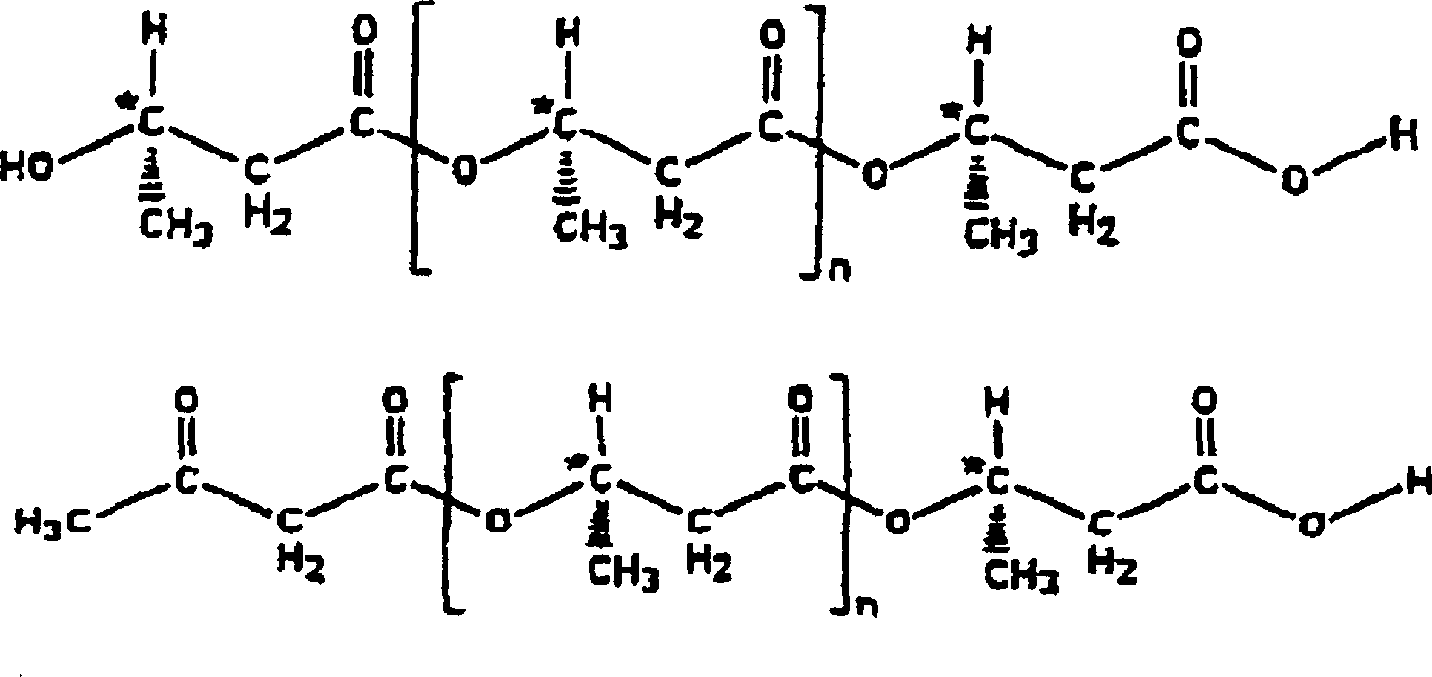
[0166] Preparation of (R)-3-hydroxybutyric acid (D-β-hydroxybutyric acid) oligomer
[0167] (R)-3-Hydroxybutyric acid (Fluka-5.0 g: 0.048 mol), p-toluenesulfonic acid (0.025 g) and benzene (100 ml) were stirred at reflux in a Dean-Stark trap apparatus for 24 hours. The reaction mixture was cooled and the benzene was distilled off in vacuo (0.5 mmHg). 4.4 g of a colorless oil were obtained and a 20 mg sample was taken to convert to its methyl ester for NMR determination of the number of repeating monomers. These experiments showed that the product was a mixture of D-β-hydroxybutyric acid oligomers with an average number of repeating units of 3.75, mainly a mixture of trimers, tetramers and pentamers, with tetramers being the most abundant. The product mixture can be dissolved in 1 N sodium hydroxide solution.
Embodiment 2
[0169] Preparation of acetoacetyl esters of (R)-3-hydroxybutyric acid oligomers
[0170] Another batch of the colorless oil from Example 1 (4.5g) was heated with diketene (3.8g) and sodium acetate (0.045g) at 60°C for 1 hour under nitrogen flow. Some more diketene (3.8g) was added and the reaction was heated for an additional hour, cooled, diluted with ether, washed with water and extracted with saturated sodium bicarbonate (5 x 100ml). The extracts were combined, washed with ether, and then acidified (dropped) with concentrated hydrochloric acid. Extraction with ethyl acetate (3 x 50ml) followed by drying over magnesium sulphate and evaporation in vacuo. A yellow solid / oil mixture (7.6 g) was obtained which was chromatographed on a silica column eluting with dichloromethane / methanol (98:2) to give the product as a bright amber oil. Isolate the faster impurity (1.6g) of flow velocity, it walks the separation column once more with carbon tetrachloride / methanol (99: 1), reclai...
Embodiment 3
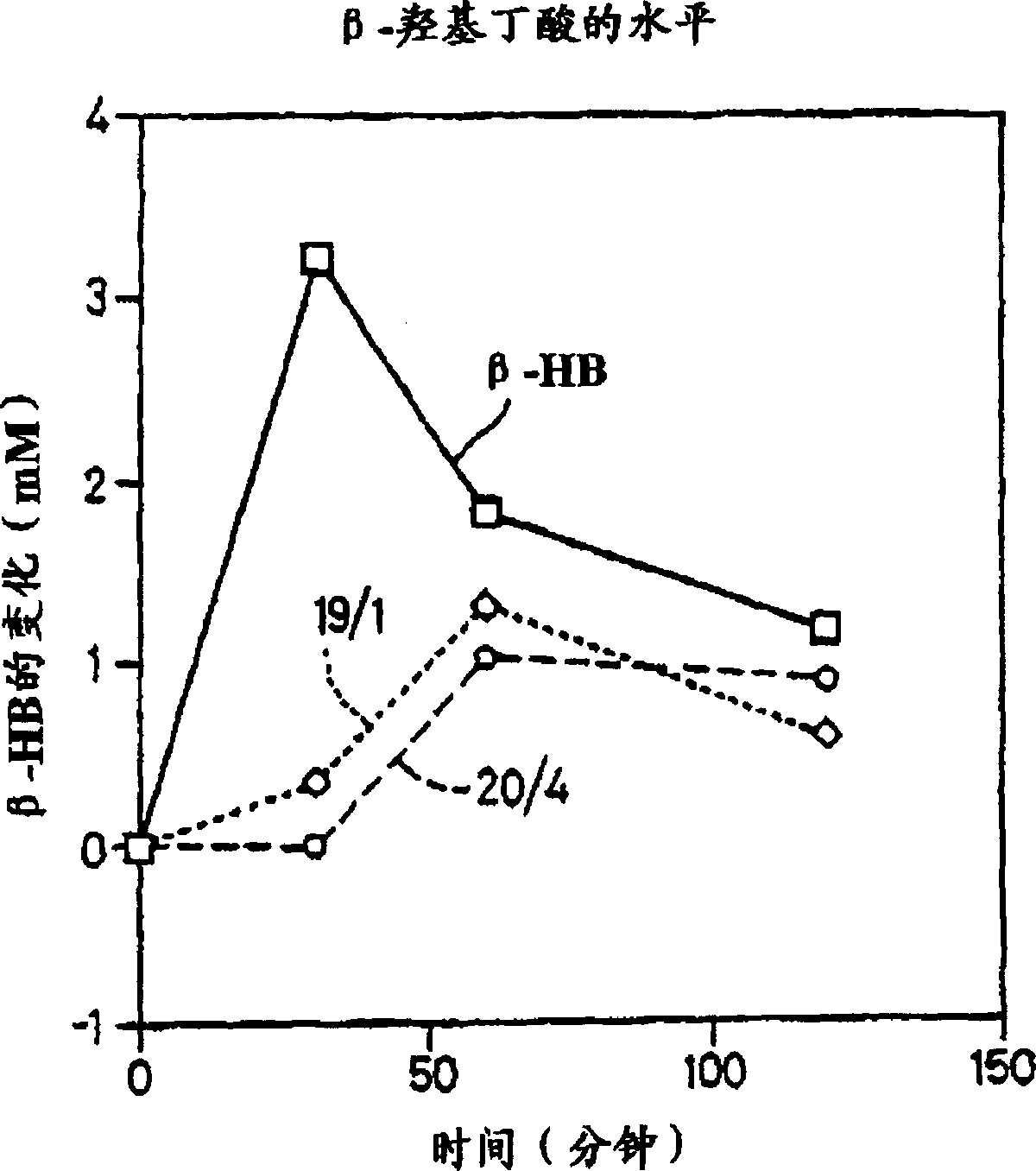
[0172] Oral administration of D-β-hydroxybutyrate, oligomers of D-β-hydroxybutyrate, and acetoacetyl D-β-hydroxybutyrate oligomers to rats
[0173] The ability of oral administration of D-β-hydroxybutyric acid and the oligomers of Examples 1 and 2 to increase blood ketone body levels was investigated in the following manner. Rats were fasted overnight and then gavaged with 4M D-[beta]-hydroxybutyrate adjusted to pH 7.74 with methylglucamine at a dose of 100 [mu]l / 100 g body weight. Use of NAD in Anal. Biochem. 131, p478-482 (1983) + / EDTA analysis method to measure the level of D-β-hydroxybutyric acid in the blood. 1.0ml of a solution prepared with 2-amino-2-methylpropanol (100mM pH 9.9, 0.094g / 10ml), NAD+ (30mM, 0.199g / 10ml) and EDTA (4mM, 0.015g / 10ml), and 4 μl of sample or D-β-hydroxybutyrate control was added to each cuvette.
[0174]Because the rats had been fasted for a period of time, initial levels of D-beta-hydroxybutyrate were already elevated compared to the fed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com