Long-acting injection capable of inhibiting abrupt release effect
An injection, long-acting technology, used in medical preparations containing active ingredients, endocrine system diseases, peptide/protein components, etc. Fast speed and other problems, to achieve a smooth and long-lasting release effect, avoid loss of protein activity, and smooth blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Take 3 g of poloxamer 407, 7.5 g of poloxamer 188, 75 μl of phenol, 19.5 g of 10 mM sterile sodium citrate buffer solution (pH 6.0), and 0.264 g of sodium chloride. Sodium chloride and phenol were dissolved in sodium citrate buffer solution, and poloxamer was slowly added to the above solution under ice bath and stirring conditions, and stored in low temperature refrigeration until a clear solution was formed. Packaged in single doses after autoclaving.
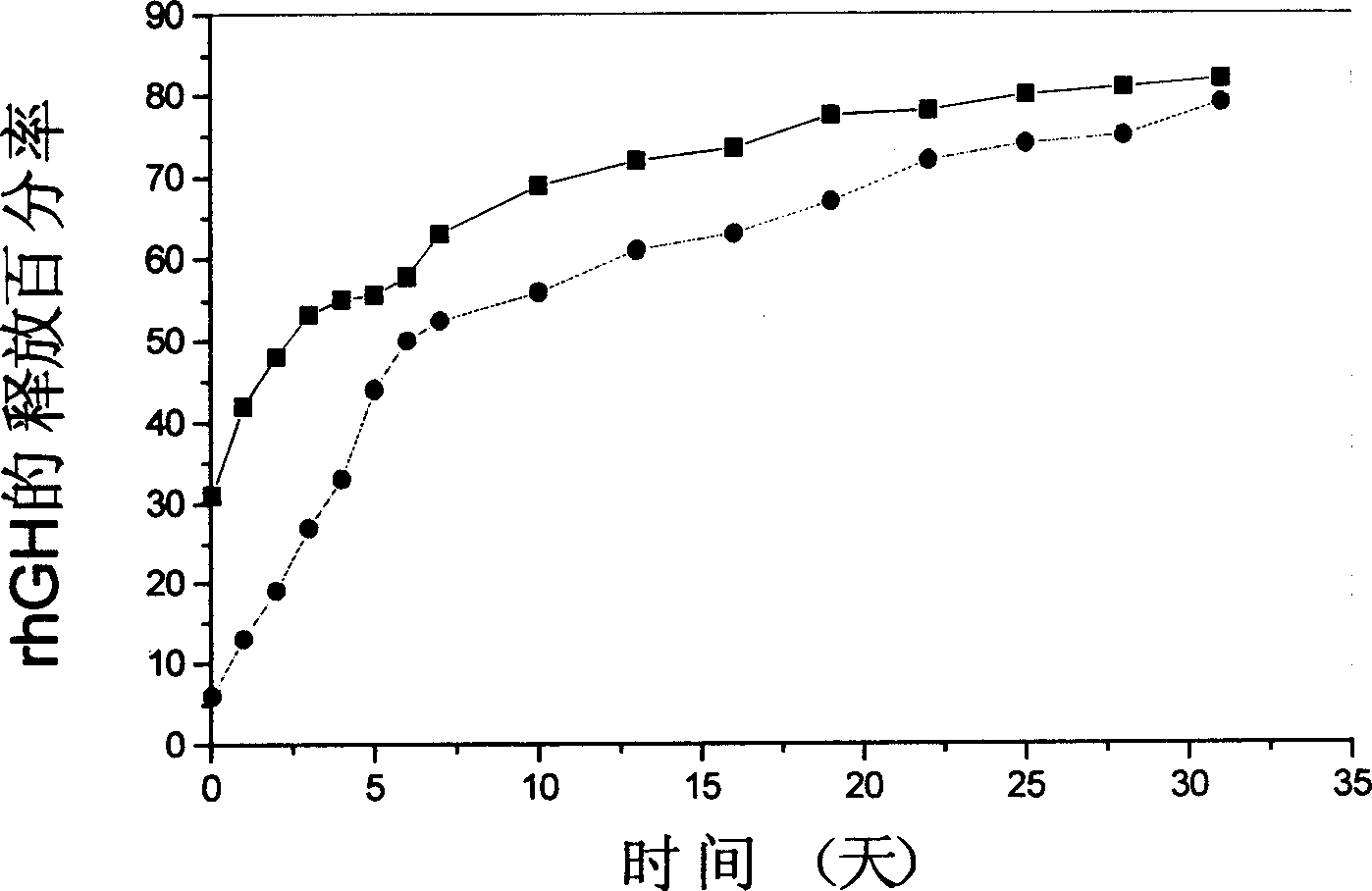
[0035] 0.6g recombinant human growth hormone (rhGH)-Zn 2+ The compound and 0.035g of zinc carbonate were added to 30ml of dichloromethane solution containing 6g of PLGA (75:25, MW 15kD), ultrasonicated for 1 minute to disperse the solid particles evenly, and then the polymer solution was added to 300ml of polyvinyl alcohol solution (6% g / ml), stirred at 2000rpm for 1min, poured the solution into 2L of distilled water and continued to stir at a lower speed for 4h to completely volatilize the organic solvent to obtain P...
Embodiment 2
[0041] Take 4.5 g of poloxamer 407, 4.5 g of poloxamer 188, 300 μl of benzyl alcohol, 19.65 g of 10 mM sterile phosphate buffer solution (pH 6.8), and 1.35 g of mannitol. Mannitol and benzyl alcohol were dissolved in phosphate buffer solution, and poloxamer was slowly added to the above solution under the conditions of ice bath and stirring, and kept in cold storage until a clear solution was formed. Packaged in single doses after autoclaving.
[0042] Dissolve 0.12g leuprolide and 0.2g mannitol in 1.5ml buffer solution, and gradually add this solution dropwise to 30ml dichloromethane solution containing 6g PLGA (50:50, MW 10kD), emulsify at 5000rpm for 1min Immediately, the suspension was sprayed into a frozen ethanol solution through an ultrasonic nozzle, and the interface of the latter was sealed with liquid nitrogen. At -70°C, use ethanol to continuously extract dichloromethane from the microspheres, and remove the ethanol by filtration to obtain microspheres with good fl...
Embodiment 3
[0045] Take 4.5 g of poloxamer 407, 4.5 g of poloxamer 188, 75 μl of phenol, 20.74 g of 10 mM sterile sodium citrate buffer solution (pH 6.0), and 0.26 g of sodium chloride. Sodium chloride and phenol were dissolved in sodium citrate buffer solution, and poloxamer was slowly added to the above solution under ice bath and stirring conditions, and stored in low temperature refrigeration until a clear solution was formed.
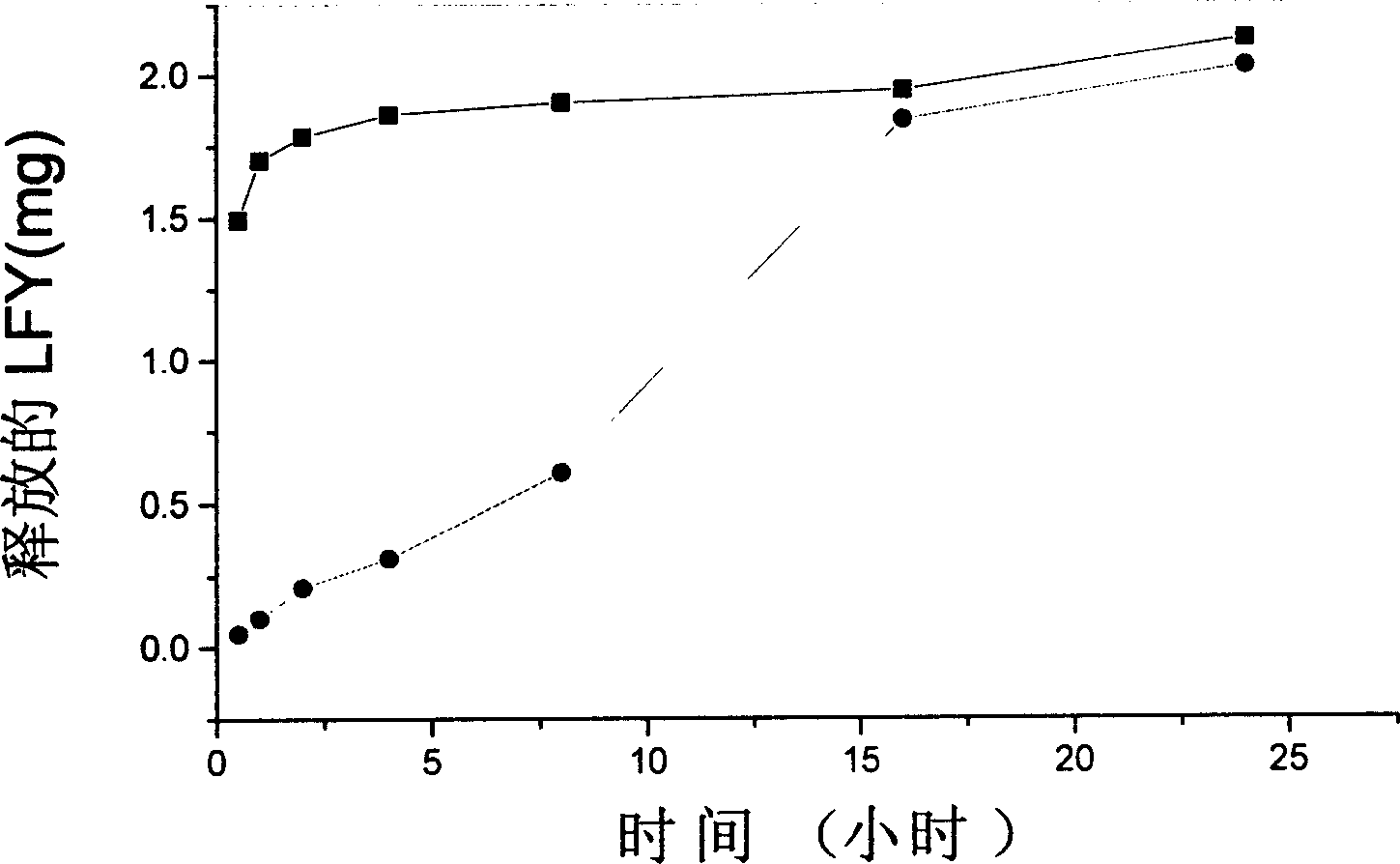
[0046] Weigh 60mg of sodium alginate and a certain amount of folic acid-dextran complex (LFY) and dissolve them in 3ml of distilled water to make a mixed solution, and add them dropwise to 50ml of rapeseed oil (containing a certain amount of Span80) at a stirring speed of 1400rpm. Stir and emulsify for half an hour, slowly pour the emulsion into 200ml containing 0.5% CaCl 2 Cured in a mixture of acetone and ethanol (1:1) for 2 hours. The precipitate was washed once with ethanol, and finally cleaned with acetone.
[0047] release test : Precisely weigh 5 mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com