Medical anticoagulant artificial material and preparing method thereof
An artificial material and anti-coagulation technology, applied in anti-coagulation treatment, medical containers, medical packaging, etc., to achieve high tensile strength, high elongation at break, and good anti-coagulation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The medical anticoagulant artificial material of this embodiment is composed of 95% by weight of polyether thermoplastic polyurethane (produced by Tianjin Polyurethane Factory) and 5% by weight of zinc oxide nanomaterial. The particles of the zinc oxide nanomaterial are distributed in the polyether thermoplastic Film materials and pipes formed in polyurethane. Among the zinc oxide nanomaterials, zinc oxide particles ≤50nm account for 97%. Zinc oxide nanomaterials are commercially available.
[0063] The preparation method is as follows: 1) The compounding of zinc oxide nano-materials with 95% by weight polyether thermoplastic polyurethane, with a Shore hardness of 85A and 5% by weight. 2) Use N, N-dimethyl acetamide (Dimethylamide) as a solvent to prepare a 7% by weight polyether thermoplastic polyurethane solution; 3) Use N, N-dimethyl acetamide (Dimethylamide) as a solvent to prepare a 7% by weight polyether thermoplastic polyurethane solution; After adding zinc oxide nan...
Embodiment 2
[0083] The method and equipment are basically the same as in Example 1, except that the material in this example is composed of 99.7% by weight of polyether thermoplastic polyurethane and 0.3% by weight of zinc oxide nanomaterials. Among the zinc oxide nanomaterials ≤50nm Zinc oxide particles account for 80%. The Shore hardness of polyether thermoplastic polyurethane is 95A.
[0084] The method is that the solvent used is N,N-dimethylacetamide (analytical purity), prepared into a 25% by weight polyether thermoplastic polyurethane N,N-dimethylacetamide solution, and stirred at 10,000 rpm for 5 Minutes, shaking for 1.5 hours, natural evaporation of the solvent at room temperature (15°C) for 8 hours, and drying at 80°C for 10 hours. The mechanical properties are average tensile strength 37.8MPa and average elongation at break 391%.
Embodiment 3
[0086] The method and equipment are basically the same as in Example 1, except that the medical anticoagulant artificial material in this example is made of 90% by weight polyester thermoplastic polyurethane (produced by Tianjin Polyurethane Factory) and 10% by weight zinc oxide It is composed of nano-materials, in which zinc oxide particles ≤50nm account for 95%. The shore hardness of polyester thermoplastic polyurethane is 95A.
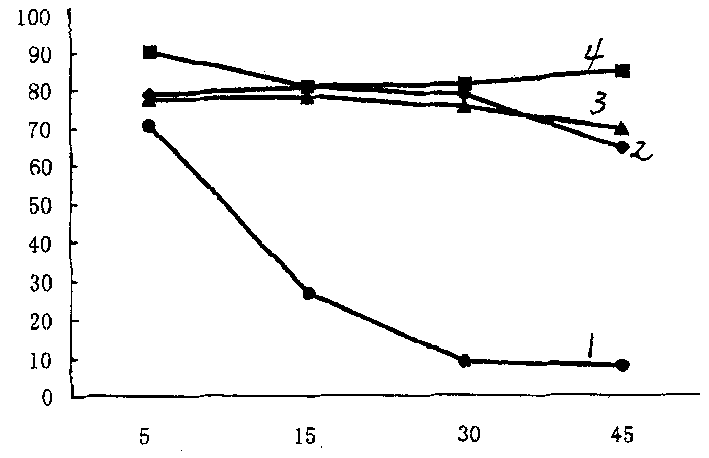
[0087] The method is to prepare a 20% by weight polyester thermoplastic polyurethane N,N-dimethylacetamide solution, stir for 120 minutes at a rate of 20,000 rpm, shake for 4 hours, and evaporate the solvent naturally at room temperature 30°C for 12 hours. Dry at 70℃ for 18 hours, the results of the dynamic anticoagulation rate test are shown in figure 1 The mechanical properties of curve 1 and curve 3 are shown in Table 3. Table 3 Mechanical properties of polyester thermoplastic polyurethane film material containing 10% by weight of zinc oxide nanomat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Shore hardness | aaaaa | aaaaa |
| Shore hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com