Novel diphenylethylene compounds
A technology of compounds and compositions, applied in the field of new stilbene compounds, can solve problems such as unknown genetic factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of 2-(4-hydroxyphenyl)-3-(3,5-dimethoxyphenyl)-sodium acrylate
[0032] To a mixture of 3,5-dimethoxybenzaldehyde (30 mM) and p-hydroxyphenylacetic acid (30 mM) was added 5 mL of acetic anhydride and 2.5 mL of triethylamine (TEA). After stirring at 130-140°C for 24 hours, the mixture was cooled to room temperature, and the reaction was terminated with 25 mL of concentrated hydrochloric acid, and CH 2 Cl 2 extraction. The organic extract was further extracted with 1N NaOH, then the NaOH extract was washed with water, the aqueous layer was acidified with conc. HCl, and washed with water to give the crude product. Acid I was obtained by recrystallization of the crude product from ethanol / water.
[0033] To obtain 1 g of decarboxylated I, 3 g of copper powder and 30 mL of quinoline were stirred and refluxed for 4 h under nitrogen. The reaction mixture was filtered, diluted with water and washed with CH 2 Cl 2 extraction. The organic layer was dried and co...
Embodiment 2
[0036] General procedure for the preparation of styrene derivatives
[0037] General procedure: To a stirred solution of Wittig's salt (1 mM) in anhydrous THF at -78°C was added potassium (bistrimethylsilyl)amide (1 mM). N 2 After stirring at -78°C for 2 hours under atmosphere, HMPA (2 mM) and aldehyde (1 mM) in THF were added and stirred at room temperature for 16 hours. The reaction was quenched with water and extracted with diethyl ether. The product was purified by flash chromatography.
Embodiment 3
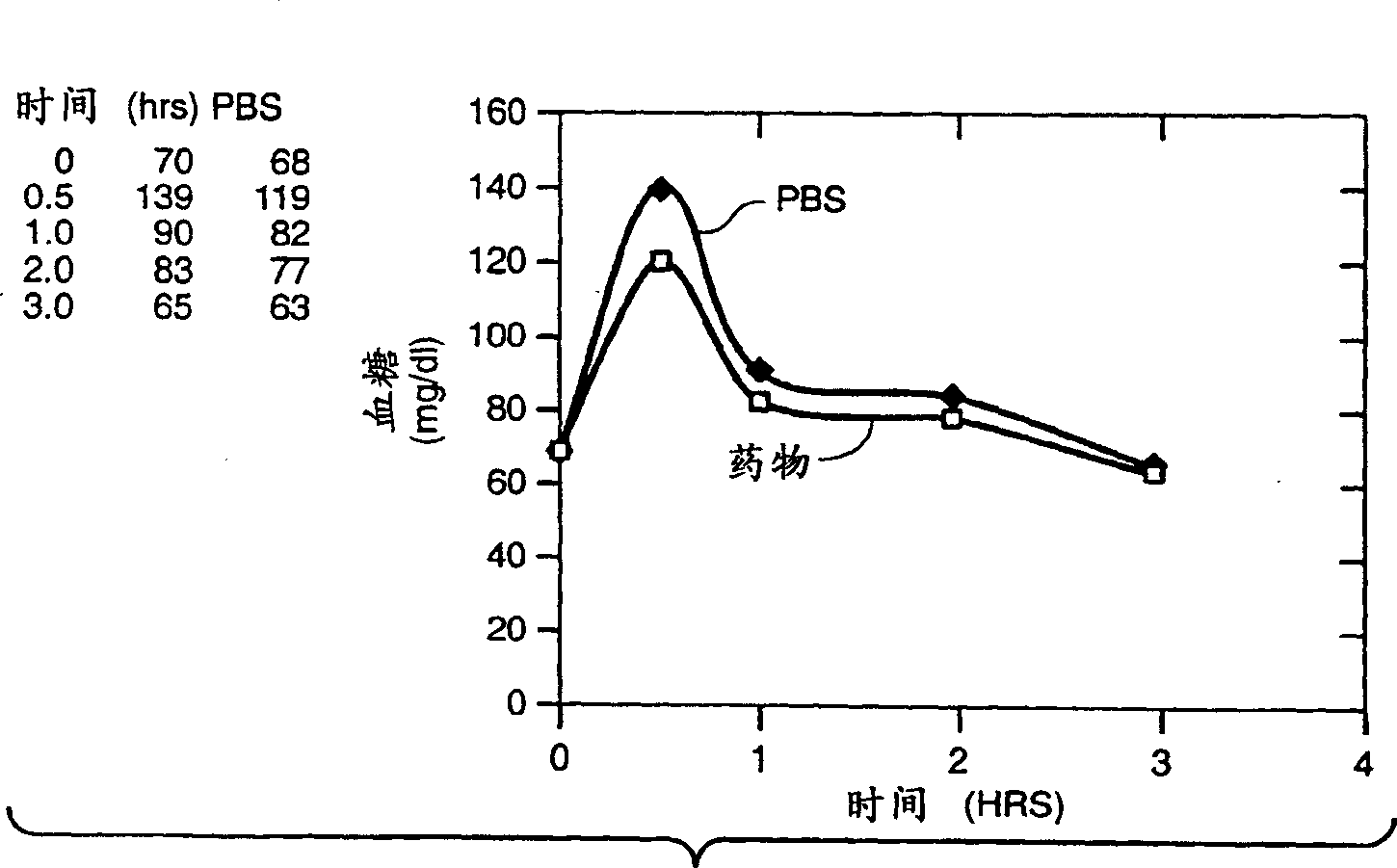
[0039]Referring to Fig. 1, STZ-induced diabetic rats were obtained by intravenous injection of streptozotocin (STZ) (40 mg / kg / body weight). Blood glucose levels were measured 72 hours after STZ injection. Rats exhibiting a fasting blood glucose level greater than 200 mg / dl were used for the experiment. The compound of Example 1 was orally administered to test rats at a dose of 20 mg / kg / body weight. At the same time, the control group was given vehicle PBS (phosphate-buffered saline). Glucose tolerance test was performed shortly after administration: glucose (2g / kg / body weight) was administered, and blood glucose levels were monitored at different time points. The results are shown in Figure 1. Between 30 and 60 minutes after dosing, the blood glucose levels of the rats administered the test compound started to decrease.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com