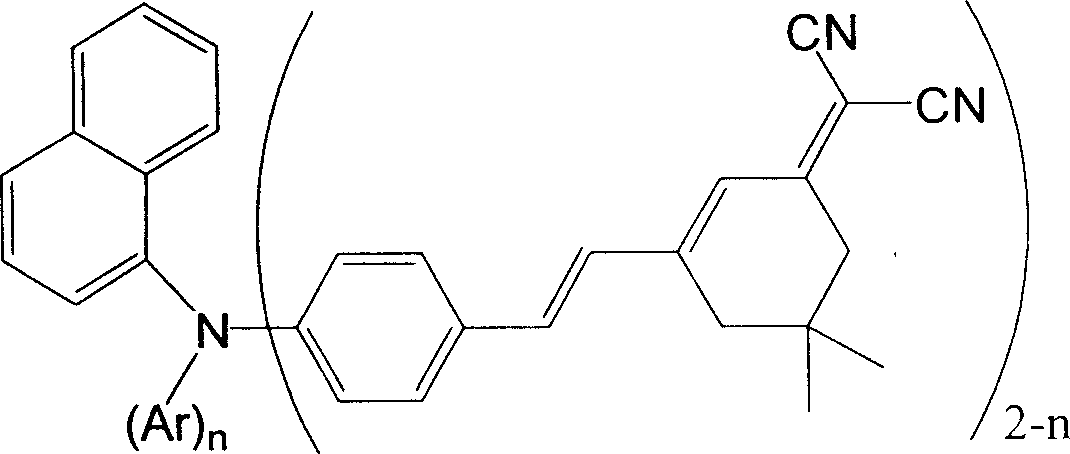
Red organic electroluminescent materials containing naphthylamine group and method for preparing same
A naphthylamine group, electroluminescence technology, applied in the field of red light-emitting materials and preparation thereof, can solve the problems of difficulty in large-scale production and application, shift of light-emitting wavelength, cumbersome preparation method, etc., and achieves reduction of self-quenching phenomenon and simple method. , the effect of good carrier transport characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
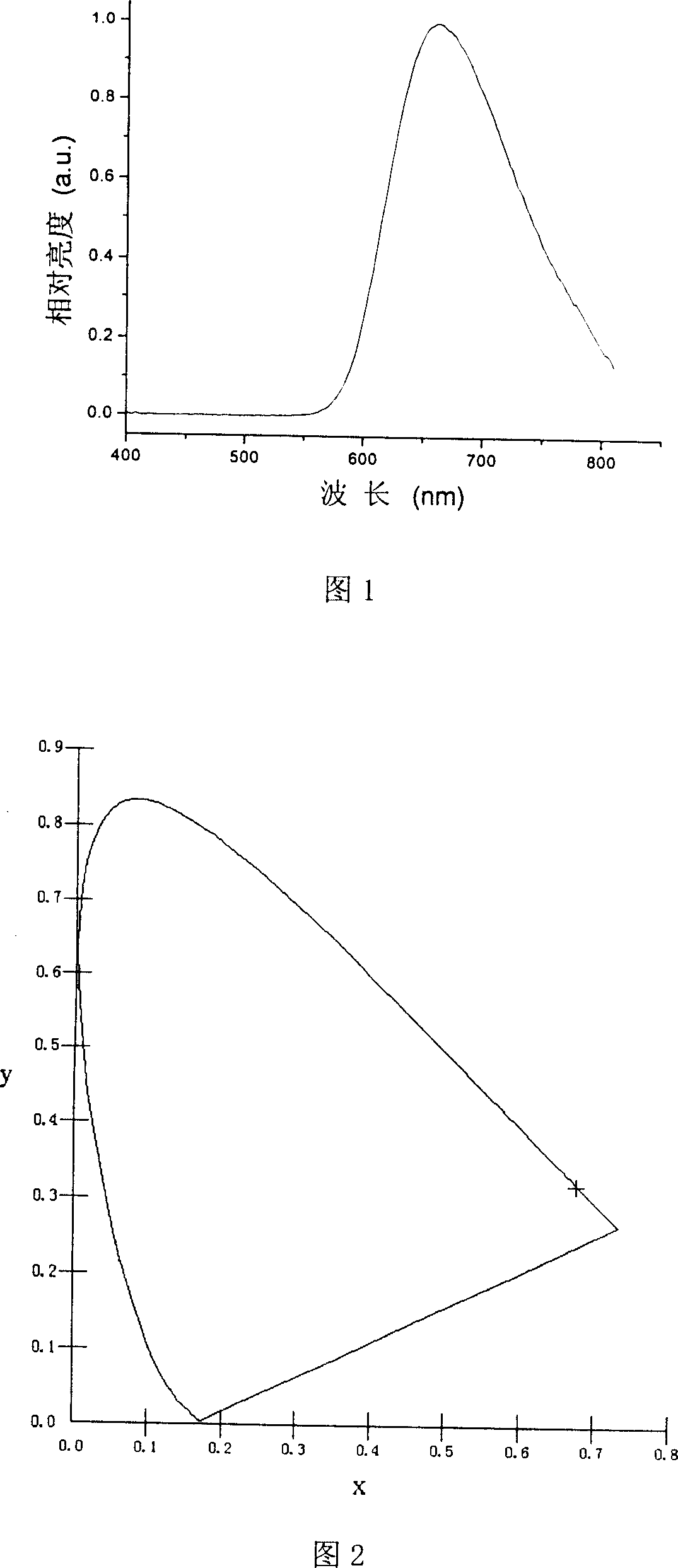
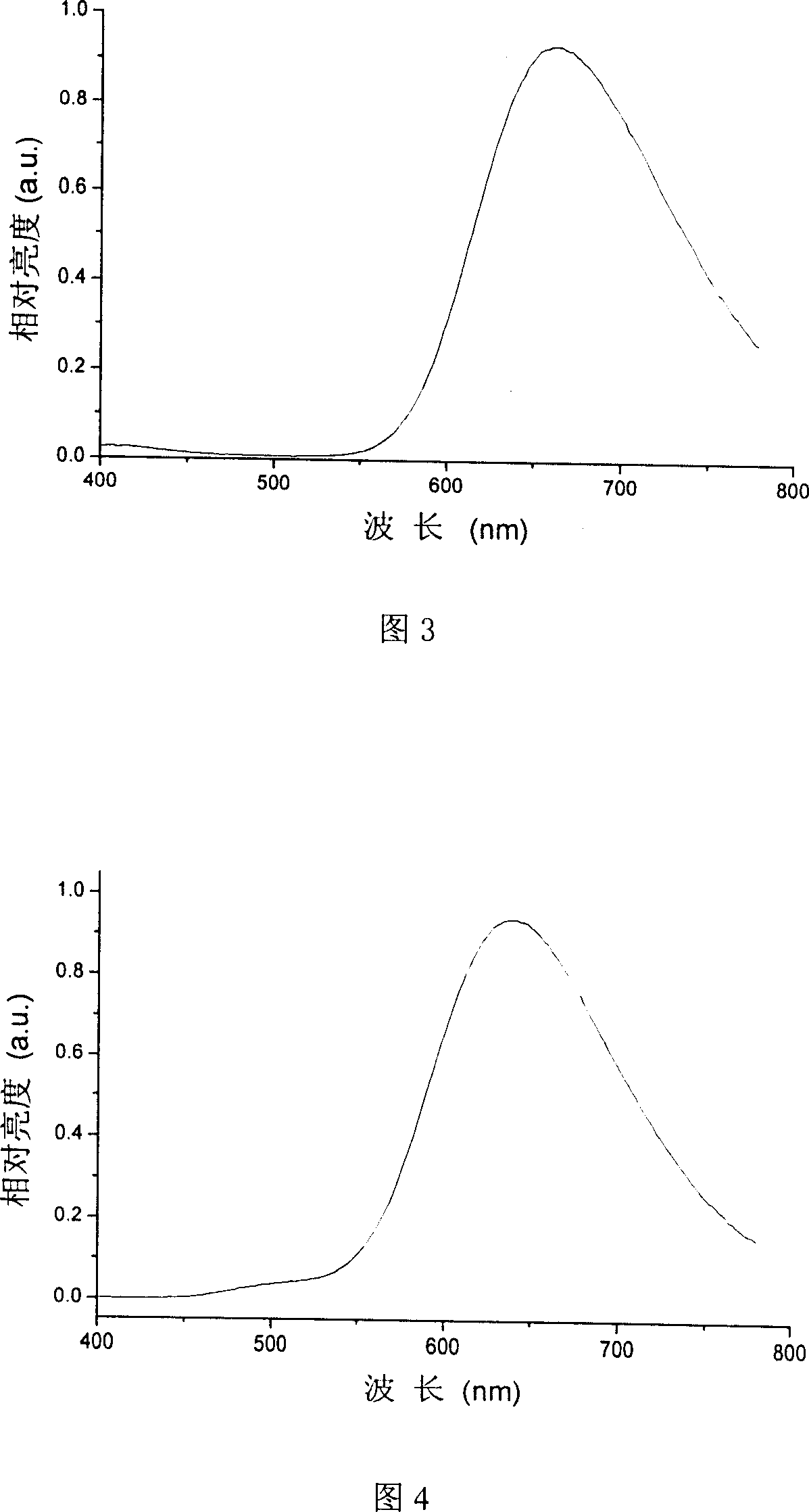
Image
Examples
Embodiment 1
[0043] Embodiment 1, the preparation of 4-(bis(1-naphthyl)amino)benzaldehyde (III-1): add 7.0g (20mmol) bis(1-naphthyl)aniline, 2.3ml (30mmol ) DMF and 30 ml 1,2-dichloroethane. The reaction flask was placed in an ice-water bath, and 2.8ml (30mmol) POCl was added dropwise under stirring 3 . After the addition, reflux in an oil bath at 95°C for 20 hours, pour into 300ml of ice water while hot, and adjust the pH of the solution to 7 with 1M NaOH solution. Extract with ethyl acetate, dry, and remove the solvent on a rotary evaporator. Purified by column chromatography (eluent: toluene) to obtain 2.70 g of light yellow product (36.2% yield).
[0044] IR (KBr, cm -1 ): 3045, 2922, 2851, 1689, 1597, 1569, 1504, 1392, 1164, 797, 774. 1 HNMR (500Hz, CDCl 3 ): δ9.76 (s, 1H), 8.0 (d, J = 8.5Hz, 2H), 7.93 (d, J = 8.3Hz, 2H), 7.81-7.79 (m, 2H), 7.59 (d, J = 8.7Hz, 2H), 7.52(t, J=7.5Hz, 2H), 7.43-7.40(m, 6H), 6.60(s(br), 2H).
Embodiment 2
[0045]Embodiment 2, the preparation of DNP-2CN: in 50ml round bottom flask, add 1.10g (8.0mmol) isophorone and 0.53g (8.0mmol) malononitrile, 0.27ml glacial acetic acid and 0.17ml acetic anhydride are dissolved in 20ml DMF, take 2.1ml and add to the reaction bottle; dissolve 1.66ml piperidine in 10ml DMF, take 2.3ml and add to the reaction bottle. Stir at room temperature for 0.5 hours, heat up to 100°C and stir for 0.5 hours, add 2.70 g (7.24 mmol) of 4-(di(1-naphthyl)amino)benzaldehyde (III-1, Example 1), and continue stirring for 0.5 hours , pour it into 200ml (containing 6ml concentrated HCl) hot water and stir while it is hot, separate the solid, stir in a mixed solution of 40ml isopropanol and 5ml water, collect the precipitated solid by suction filtration, recrystallize in acetonitrile to obtain a red crystal product 2.50 g (63.8% yield). Melting point: 250°C.
[0046] IR (KBr, cm -1 ): 3035, 2924, 2853, 2217, 1687, 1602, 1586, 1554, 1521, 1502, 1392, 1304, 1290, 117...
Embodiment 3
[0051] Embodiment 3, the preparation of 4-(phenyl (1-naphthyl) amino) benzaldehyde (III-2): add 4.56g (15.5mmol) (1-naphthyl) diphenylamine, 1.8ml in 100ml eggplant-shaped bottle (23mmol) DMF and 40ml 1,2-dichloroethane. The reaction flask was placed in an ice-water bath, and 2.1ml (23 mmol) POCl was added dropwise under stirring 3 . After the addition, reflux in an oil bath at 95°C for 1 hour, pour into 200ml of ice water while hot, and adjust the pH of the solution to 7 with 1M NaOH. Extract with ethyl acetate, dry, and remove the solvent on a rotary evaporator. Purification by column chromatography (eluent: toluene / petroleum ether (60-90° C.) = 1 / 1) gave 3.26 g of a light yellow product (65.1% yield).
[0052] IR (KBr, cm -1 ): 3035, 2851, 1687, 1602, 1586, 1504, 1491, 1391, 1249, 1223, 1163, 774, 752, 695.
[0053] 1 H NMR (500Hz, CDCl 3 ): δ9.78(s, 1H), 7.92(d, J=8.2Hz, 1H), 7.86(t, J=8.2Hz, 2H), 7.64(d, J=8.7Hz, 2H), 7.53-7.48 (d+d, 2H), 7.43-7.38(d+d, 2H), 7.31(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com