Method for preparing megakaryocytic preparation by amplifying macronucleus ancestral cell and mature megacaryocyte and use
A technology of mature megakaryocytes and megakaryotic progenitor cells, applied in biochemical equipment and methods, extracellular fluid diseases, medical preparations containing active ingredients, etc., can solve the problems of poor clinical applicability and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: In vitro induction and expansion of megakaryocytes
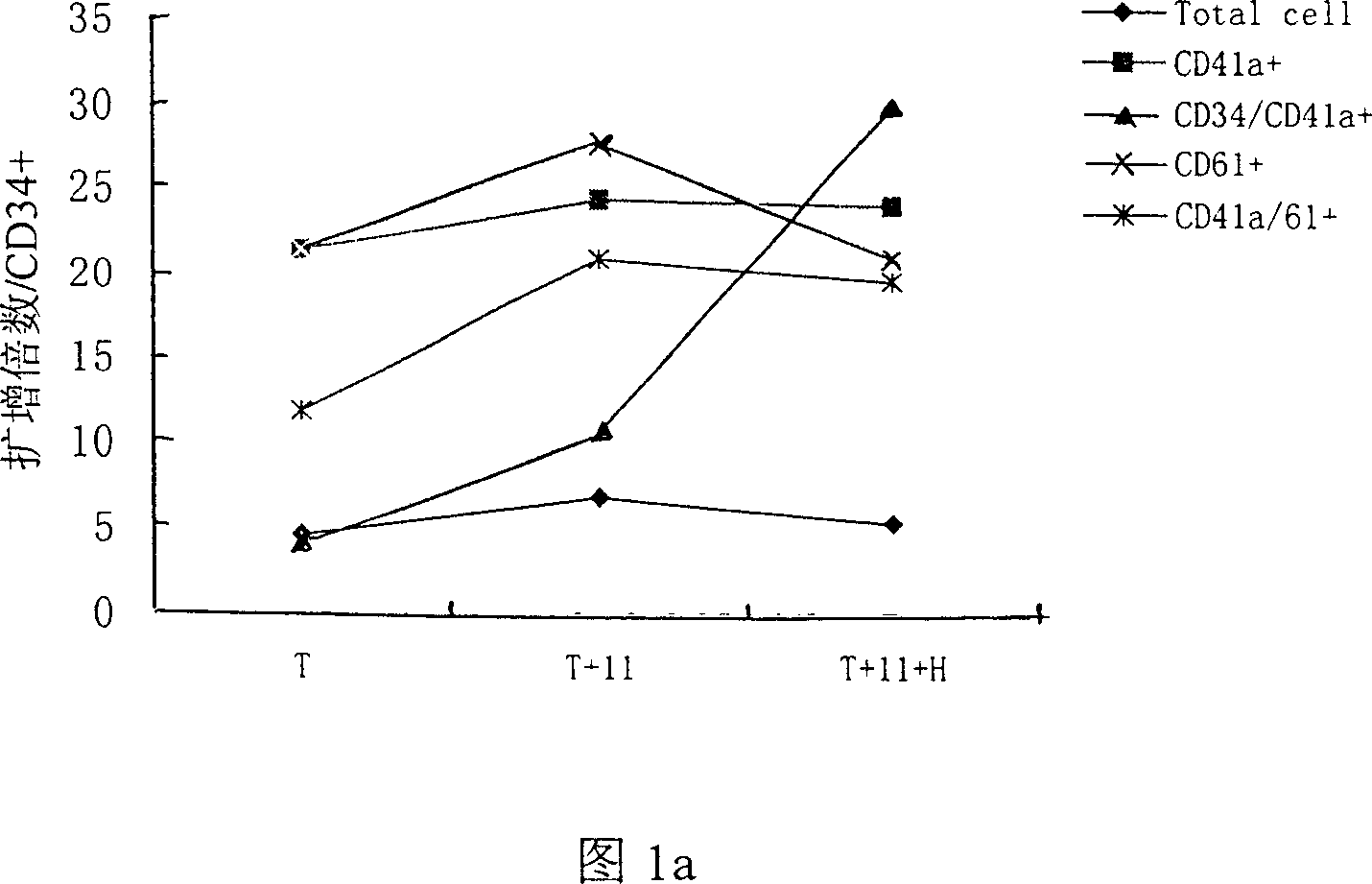
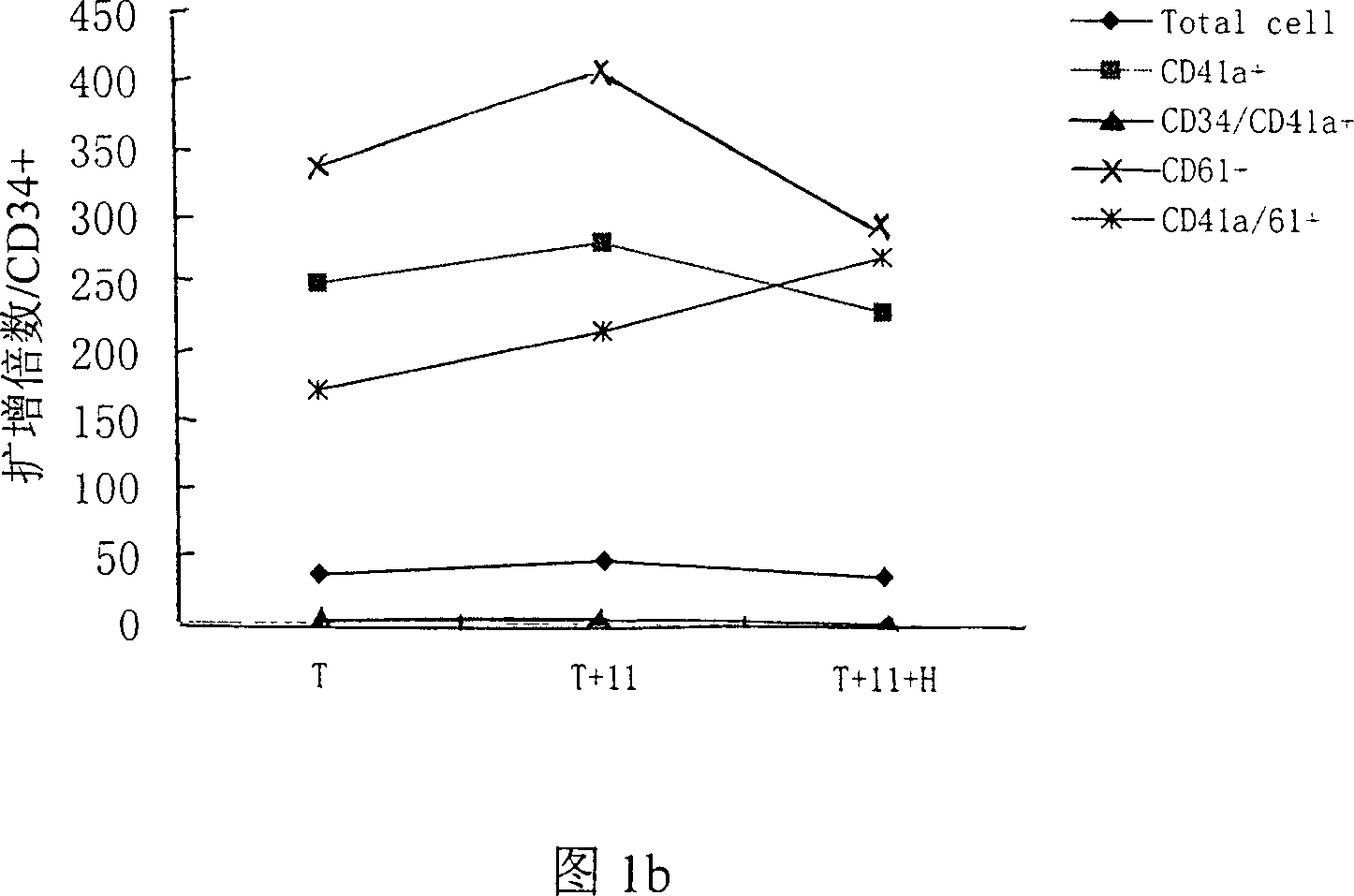
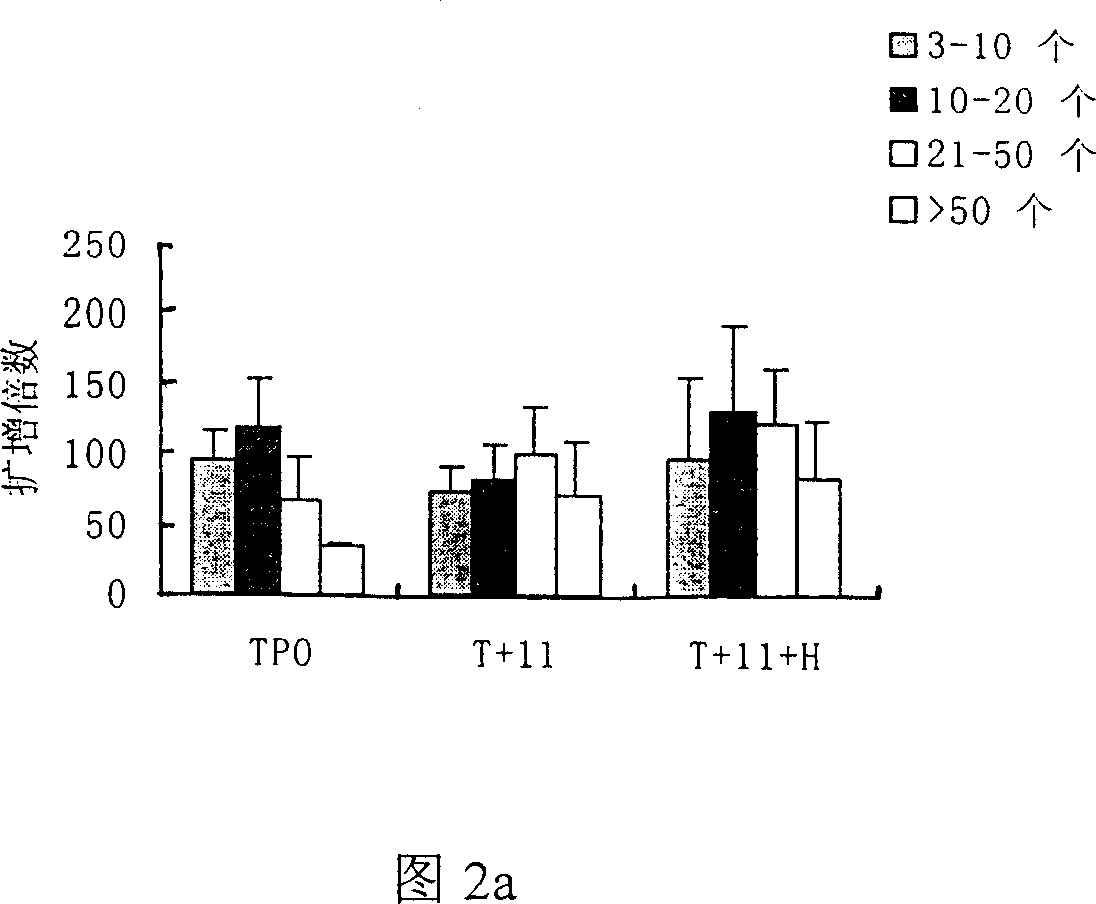
[0042] Freshly isolated cord blood CD34+ cells (1×10 5 / ml) inoculated in the serum-free medium containing thrombopoietin (TPO) of the present invention+recombinant human interleukin-11 (rhIL-11)+heparin (Heparin) in the final volume, wherein containing TPO: 50ng / ml ml, IL-11: 50ng / ml, Heparin: 25IU / ml, at 37°C, 5% CO 2 Cultured in an incubator in a humid environment, half of the medium was changed every 3.5 days, and various analyzes were performed on the 7th, 10th, and 14th day. At the same time, two serum-free culture expansion methods of TPO alone (50ng / ml) and TPO (50ng / ml)+IL-11 (50ng / ml) combination were used as controls to evaluate the efficacy of TPO+IL-11+heparin combination. The effect of serum culture expansion system on the preparation of megakaryocyte products. See Table 1, Table 2 and Figure 1a, Figure 1b, Figure 2a, Figure 2b, Figure 3a, Figure 3b, and Figure 4 for the results of the tota...
Embodiment 2
[0055] Example 2. Application of megakaryocyte products expanded in vitro from umbilical cord blood CD34 positive cells
[0056] 1) In this example, umbilical cord blood from normal delivery was taken, and CD34 was separated by magnetic bead affinity column (MACS) separation method + Cells were added to serum-free medium containing TPO+IL-11+Heparin for directional expansion for 7 days, and megakaryocytes were collected to make 1×10 7 / 100ul spare.
[0057] 2) Non-obese diabetic / severe combined immunodeficiency mice (NOD / SCID) were randomly divided into two groups after Cs source irradiation (275cGy), one group was tail vein injection of PBS group, and the other group was tail vein injection of amplification The above megakaryocyte groups were all injected within 4 hours after irradiation, and transplanted 1×10 7 / 100ul of megakaryocytes, on the 7th day, 14th day, and 21st day after transplantation, the peripheral blood of the mice was measured, and the flow cytometry was us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com