Specific protein of SARS virus, clinical detection method and kit
A technology of pneumonia virus and detection method, applied in the field of human medical and health, to achieve the effect of high accuracy and accurate diagnosis method and kit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Preparation of protein:
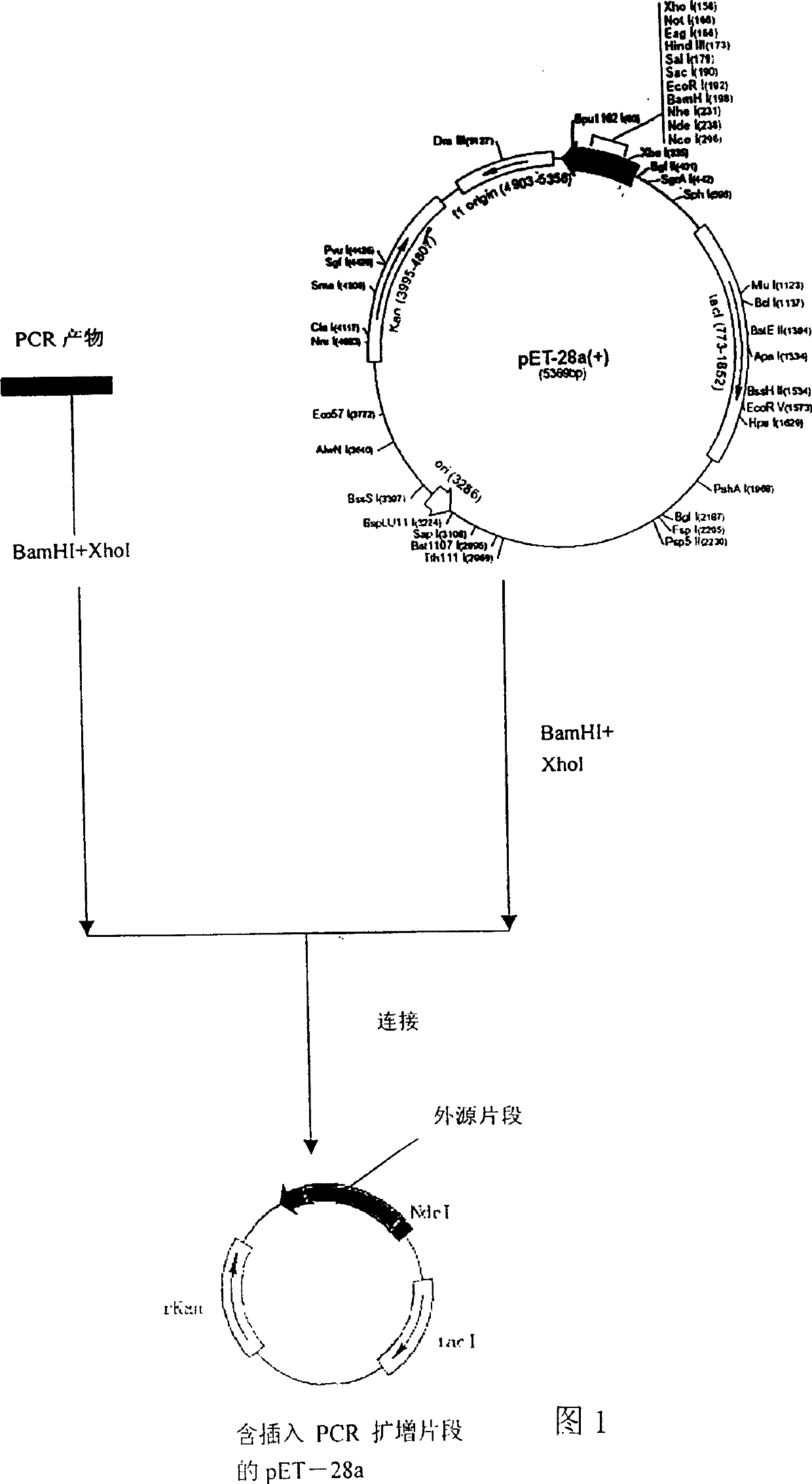
[0035] A. Design corresponding PCR primers, and add bases corresponding to the corresponding number of amino acid residues to the PCR primers used. One of the primers is as follows: AlL: CGGGATCCGAACTTTAAAATCTGTGTAGC AlR: CCGCTCGAGATTTCTGCAACCAGCTCAAC
[0036] Extract RNA and amplify the DNA fragments encoding the above proteins from clinical atypical pneumonia virus standard strains respectively; the method of extracting RNA can adopt the method introduced in the general molecular biology experiment manual, such as "molecular cloning", or use commercial reagents box, such as Trizol R , according to the instructions, then add reverse primer and reverse transcriptase to synthesize cDNA; use commercial kits, such as Invitrogen TM The company's SuperScript kit synthesizes cDNA according to the instructions; then PCR technology is used to amplify the DNA fragments encoding the above proteins. The composition of the PCR reaction system is a gen...
Embodiment 2
[0053] Example 2, Double Antigen Sandwich Method for Detection of SARS Antibody
[0054] Add the coating solution diluted with 0.02M pH9.6 carbonate buffer (CB) to the optimum concentration in advance on the 96-well microwell reaction plate, let stand at 4°C for 24 hours, add 0.1% TW-20 0.02M pH7.4 PBS washing solution (PBS-T) was added to each well, discarded after standing for a few seconds, and washed twice. Add 200 μl of 0.02M pH7.4 phosphate buffer saline (PBS) with 1% bovine serum albumin (BSA) and 1% skim milk powder to each well, and block for 2 hours at 4°C. Discard the blocking solution, dry at room temperature, seal the plate with an aluminum thin bag, and store at 4°C until use. Add the enzyme-labeled antigen corresponding to the SARS coronavirus antigen diluted with the optimized concentration in advance with the enzyme-labeled antigen diluent (20% goat serum, 1% casein, 0.02MPBS, pH7.4) in the well of the microwell reaction plate, 50 μl per well, then add 15 se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com