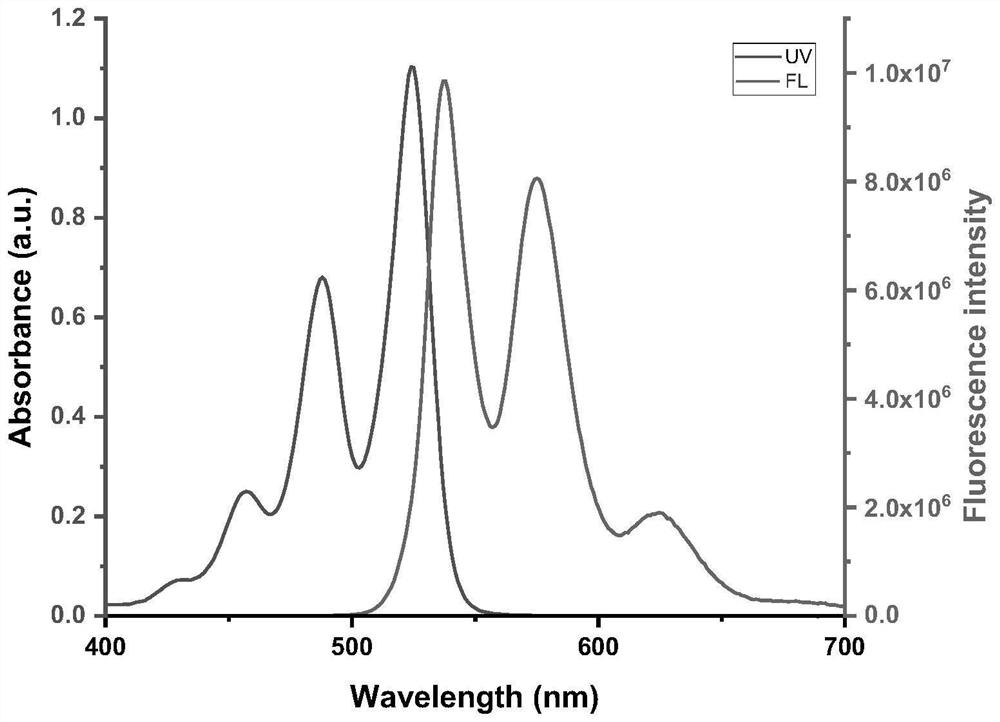
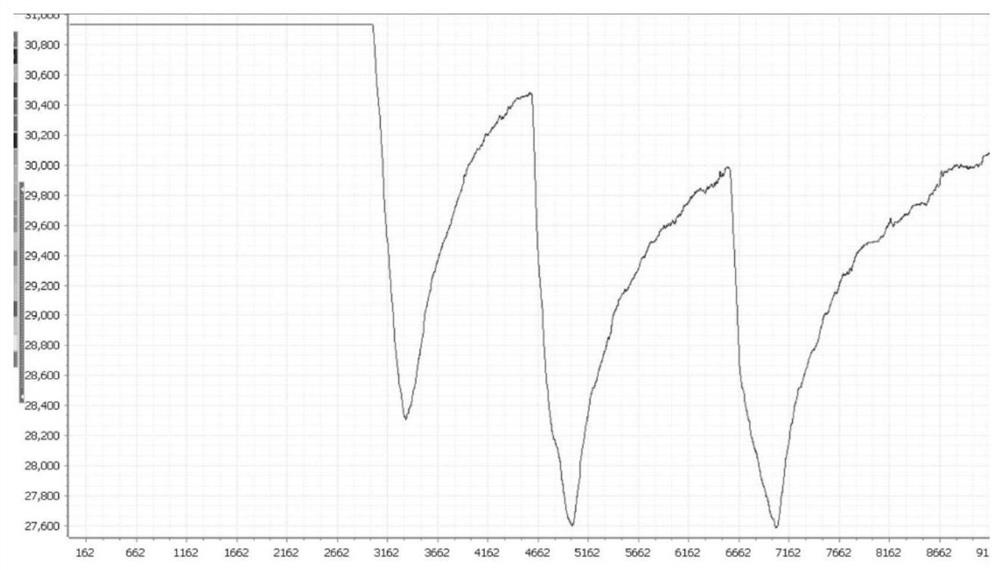
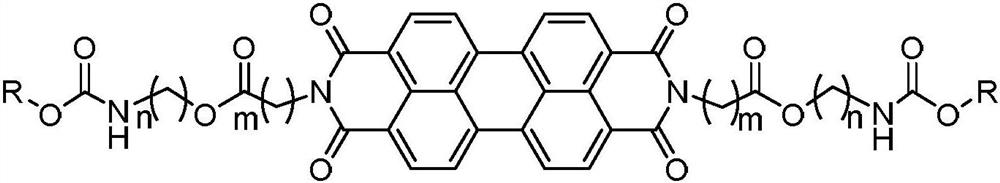
Steroid-perylene diimide compound, preparation method thereof, and preparation and application of fluorescent film of steroid-perylene diimide compound
A perylene diimide and compound technology, applied in the field of steroid-perylene diimide compounds, can solve the problems of high sensitivity requirements for instrument consumables, reagent pollution, preparation conditions, and expensive detection, and achieve excellent repeated use times, high Fluorescence quantum yield, the effect of rapid and sensitive detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Under nitrogen protection, dissolve 10g β-sitosterol in 50ml of anhydrous tetrahydrofuran, add 12.4g of anhydrous sodium carbonate, cool to 0°C, and then dissolve 13.9g of triphosgene in 20ml of anhydrous tetrahydrofuran, place in ice The solution was added dropwise at a rate of 20 drops / min under the bath. After the dropwise addition was completed, the reaction was carried out in an ice bath for two hours, and then the reaction was stirred at room temperature for 12 hours. After the reaction, the reactant was diluted with hexane and subjected to suction filtration. The filtrate was collected and concentrated under reduced pressure. The crude product was separated by a petroleum ether / dichloromethane (4:1) chromatographic column to prepare 8.8 g of a light yellow oily compound, i.e. β-valley Sterol chloroformate, ESI-MS (m / z): 477.27 [M+H]+. Its reaction equation is as follows:
[0034]
[0035] 2) Under nitrogen protection, dissolve 0.67g of ethanolamine in 20mL...
Embodiment 2
[0047] 1) Under nitrogen protection, dissolve 10g of stigmasterol in 50ml of anhydrous tetrahydrofuran, add 12.4g of anhydrous sodium carbonate, cool to 0°C, and then dissolve 13.9g of triphosgene in 20ml of anhydrous tetrahydrofuran, in an ice bath The solution was added dropwise at a rate of 20 drops / min. After the dropwise addition was completed, the reaction was performed in an ice bath for 2 hours, and then the reaction was stirred at room temperature for 10 hours. After the reaction, the reactant was diluted with hexane and subjected to suction filtration, the filtrate was collected and concentrated under reduced pressure, and the crude product was separated by a petroleum ether / dichloromethane (5:1) chromatographic column to prepare 9.3 g of a transparent oily compound, namely stigmasterol chloromethyl acid ester. ESI-MS (m / z): 475.61 [M+H]+. Its reaction equation is as follows:
[0048]
[0049] 2) Under nitrogen protection, dissolve 0.67 g of ethanolamine in 20 m...
Embodiment 3
[0055] 1) Under the protection of nitrogen, dissolve 10g of ergosterol in 50ml of anhydrous tetrahydrofuran, then add 12.4g of anhydrous sodium carbonate, cool to 0°C, then dissolve 13.9g of triphosgene in 20ml of anhydrous tetrahydrofuran, in an ice bath The solution was added dropwise at a rate of 20 drops / min. After the dropwise addition was completed, the reaction was performed in an ice bath for 2 hours, and then the reaction was stirred at room temperature for 12 hours. After the reaction, the reactant was diluted with hexane to carry out suction filtration, the filtrate was collected and concentrated under reduced pressure, and the crude product was separated by a petroleum ether / dichloromethane (4:1) chromatographic column to prepare a pale yellow oily compound 6.7g, namely ergosterol chloride. formate. ESI-MS (m / z): 459.60 [M+H]+. Its reaction equation is as follows:
[0056]
[0057] 2) Under the protection of nitrogen, dissolve 0.67g of ethanolamine in 20mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com