Method for efficiently preparing chiral boride based on mobile phase
A boride and mobile phase technology, applied in the field of efficient preparation of chiral borides based on mobile phase, can solve the problems of harsh reaction conditions, environmental pollution, high cost, etc., and achieve mild reaction conditions, improved preparation efficiency, and short reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] This embodiment provides a cellulose-supported Cu 2 O / TiO 2 The preparation method comprises the following steps:
[0057] P1. Add 20mL n-butyl titanate and 70mL absolute ethanol into the beaker, stir for 20min to obtain a yellow clear solution a; add 4mL glacial acetic acid, 20mL distilled water and 70mL absolute ethanol into the beaker, stir for 20min, and add dropwise 1mol / L HCl was adjusted to pH 3 to 4 to obtain solution b;
[0058] P2. Mix solution a and solution b obtained in step P1, stir in a water bath at 50 °C for 1 h to obtain a white gel, then place the white gel in an oven at 80 °C for 24 h to obtain yellow crystals, grind to obtain yellow powder, and then put The yellow powder was calcined at 600 °C for 2 h to obtain TiO 2 powder;
[0059] P3. Take 0.0802g NaOH and add it to 20mL distilled water, stir and dissolve to obtain NaOH solution; add 1mmol Cu(OAc) 2 Add to NaOH solution, stir and mix; then add 1 mmol of TiO obtained in step P2 2 And stir fo...
Embodiment 2
[0071] The present embodiment provides a method for efficiently preparing a chiral boride based on a mobile phase, comprising the following steps:
[0072] S1. The cellulose obtained in step P4 of Example 1 is loaded with Cu 2 O / TiO 2 The suspension was passed into the microchannel reactor until the entire reaction channel was filled, and then the microchannel reactor was placed in a drying oven at 150 °C to dry;
[0073] S2. Add 0.20 mmol α, β unsaturated ketone, 0.4 mmol biborate pinacol ester and 0.002 mmol ligand (R, S)-josiphos to 1 mL of toluene to pre-dissolve to obtain mixed solution c; The pressure of 120KPa continuously feeds distilled water and mixed solution c into the microchannel reactor, and the volume ratio of distilled water and mixed solution c is 9:1;
[0074] The α,β unsaturated ketone of this embodiment is chalcone (I-1), and the reaction formula in step S2 is as follows:
[0075]
[0076] S3. Collect the reaction solution at the sample outlet, extra...
Embodiment 3
[0088] This example is basically the same as Example 2, except that the α,β unsaturated ketone in this example is 3-(4-methylphenyl)-1-phenyl-2-propen-1-one ( I-2), the reaction formula is as follows in step S2:
[0089]
[0090] The chiral boride II-2 obtained by the preparation method in this example is 41.53 mg, the yield is 93%, and the ee value reaches 98%.
[0091] The reaction formula for the conversion of chiral boride II-2 to chiral hydroxyl compound III-2 is as follows:
[0092]
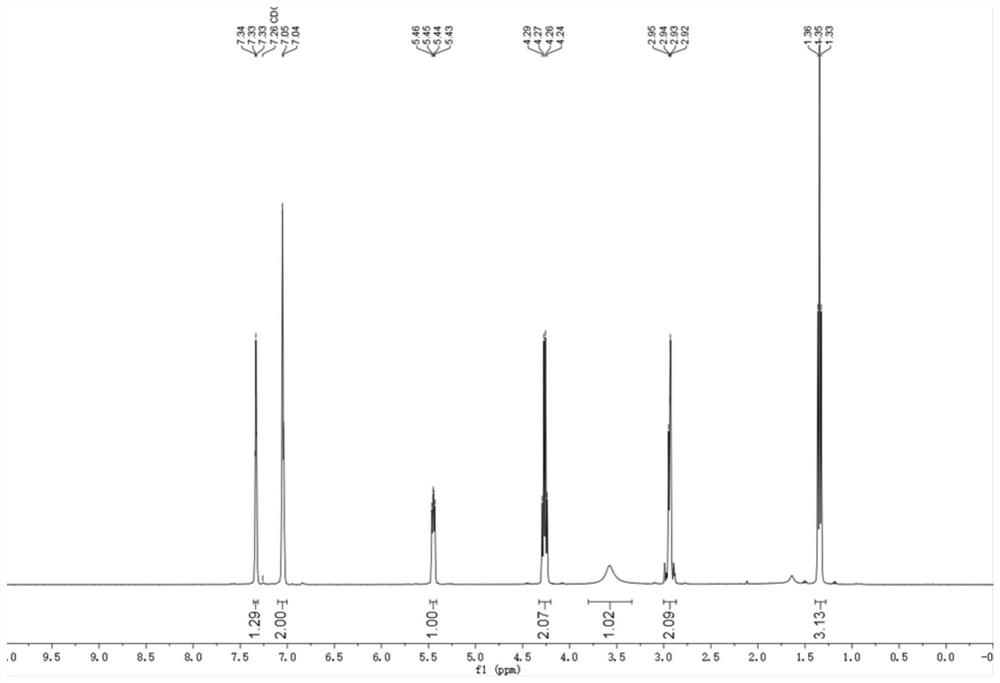
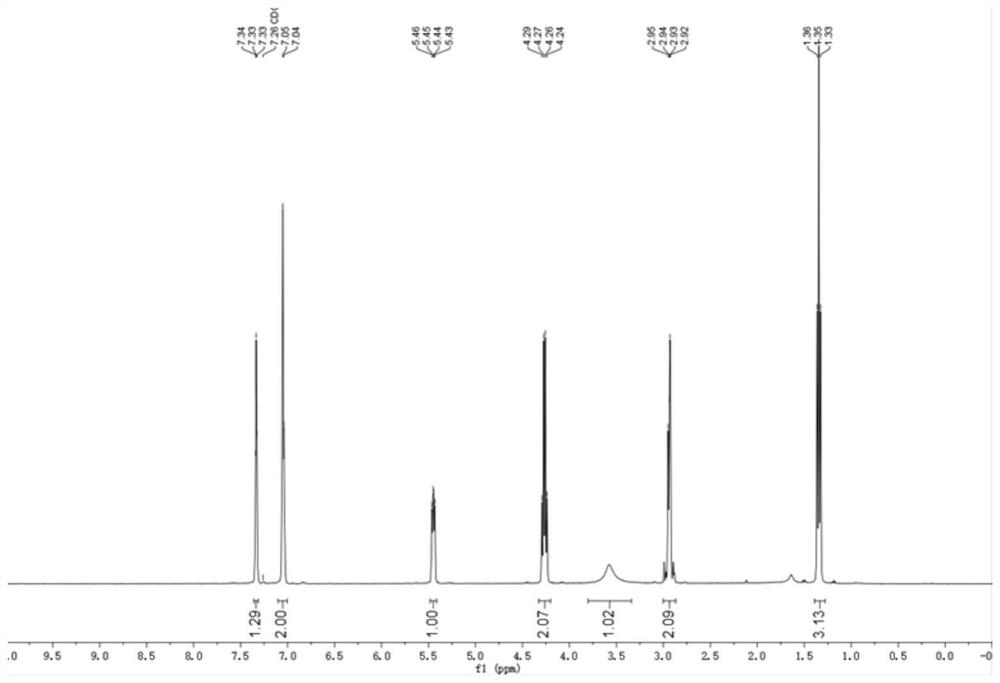
[0093] The H NMR and C NMR spectra of the chiral hydroxyl compound III-2 in this example are as follows:
[0094] 1 H NMR (400 MHz, Chloroform-d); δ=7.98–7.94 (m, 2H), 7.65–7.58 (m, 1H), 7.50–7.47 (m, 2H), 7.34–7.31 (m, 2H), 7.20– 7.18 (m, 2H), 5.33–5.29 (m, 1H), 3.63 (br, 1H), 3.40–3.30 (m, 2H), 2.34 (s, 3H).
[0095] 13 C NMR (100 MHz, Chloroform-d); δ=200.3, 140.0, 137.8, 136.5, 133.6, 129.2, 128.7, 128.2, 125.9, 69.9, 47.4, 21.5.
[0096] The high-performance liquid phase da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com