Eutectic of apixaban and fumaric acid as well as preparation method and application thereof
A technology of apixaban and fumaric acid, which is applied in the field of co-crystals formed by apixaban and fumaric acid and its preparation, can solve the problem of low crystal form dissolution rate, limited clinical application of new product development, and difficulty in obtaining ideal It can improve the bioavailability, the best purification and impurity removal effect, and the effect of easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1: Preparation of co-crystals of apixaban and fumaric acid
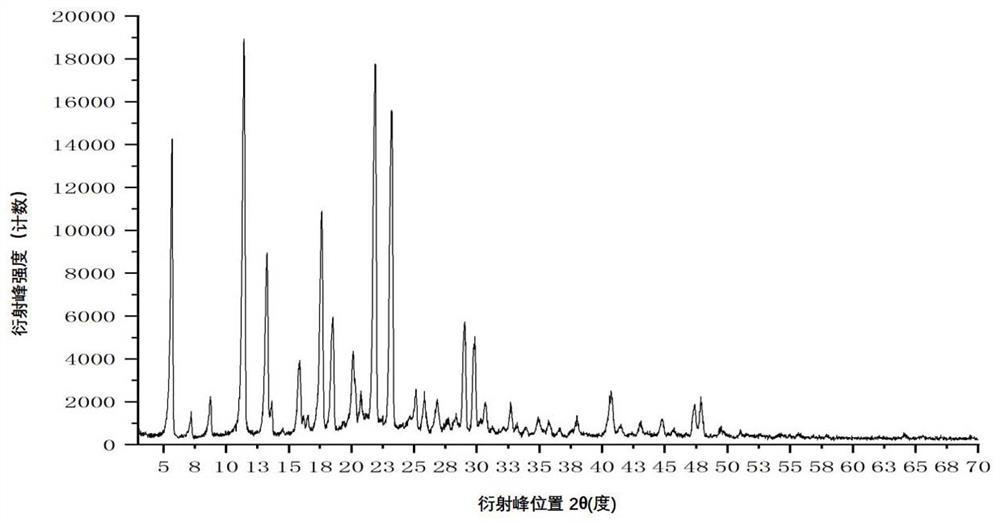
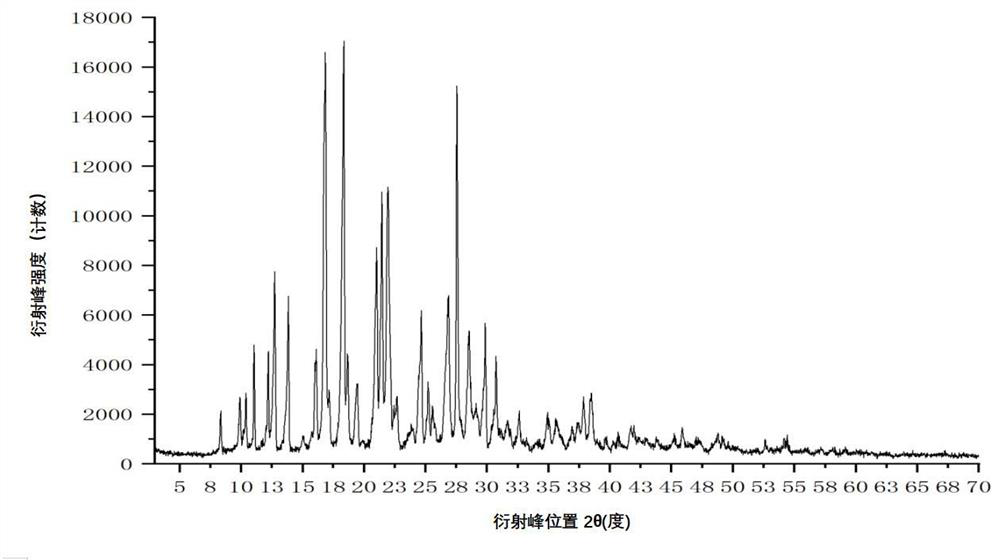
[0117] Add 400 mL of acetonitrile to a 1000 mL three-necked bottle, and add 40.00 g of crude apixaban (Suzhou Capwell Pharmaceutical Technology Co., Ltd., HPLC: 99.08%, see details for details). Figure 8 ), turn on stirring, take another 250mL beaker, add 5.05g fumaric acid and 100mL purified water, stir and dissolve, add the purified aqueous solution of fumaric acid to the above three-necked flask, heat the system to reflux, and cool down to 20 after the system is dissolved. ℃~30℃, stir for 1~2 hours and then filter, collect the solid and dry it under vacuum at 40℃~60℃ for 4 hours to obtain 40.10g of white solid, the melting point is 242.5~244.5℃, the purity detected by HPLC is: 99.67%, see details for details Figure 9 .
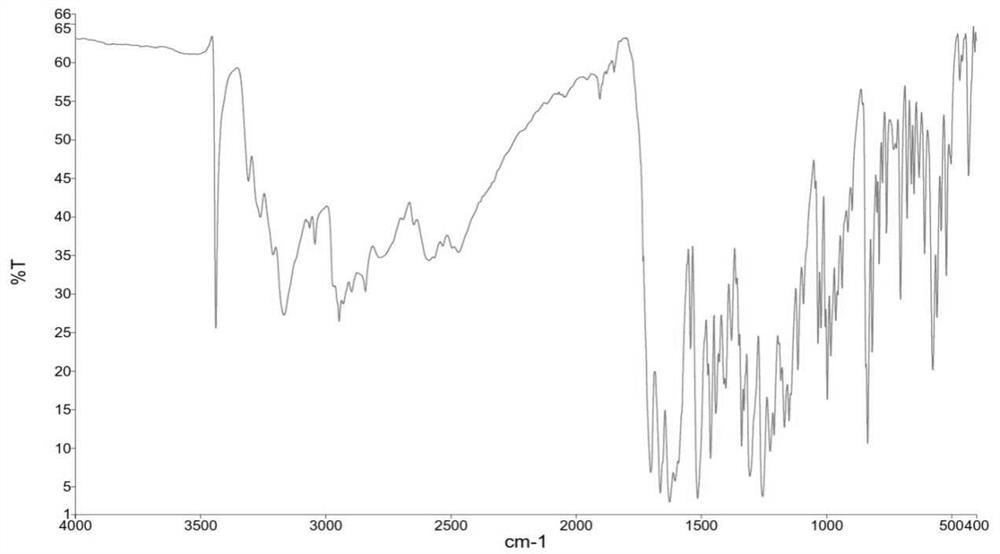
[0118] By X-ray powder diffraction (XRPD), infrared spectroscopy (IR), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), hydrogen nuclear magnetic resona...
Embodiment 2
[0119] Example 2: Preparation of co-crystals of apixaban and fumaric acid
[0120] Add 50 mL of acetonitrile to a 100 mL three-necked flask, add 5.00 g of apixaban, turn on stirring, add 0.13 g of fumaric acid and 10 mL of purified water to another 50 mL beaker, stir to dissolve, and add the prepared purified aqueous solution of fumaric acid to the above three-necked flask. In the bottle, the system was heated to 50°C, the system was dissolved and then cooled to 30°C~40°C, and the mixture was stirred for 1~2 hours, filtered, and the collected solid was vacuum-dried at 20°C~40°C for 8 hours to obtain 0.37g of white solid. The melting point is 243.0~244.5°C, and it is confirmed to be a new crystal form of apixaban. The purity detected by HPLC is: 99.73%.
Embodiment 3
[0121] Example 3: Preparation of co-crystals of apixaban and fumaric acid
[0122] Add 50 mL of acetonitrile to a 100 mL three-necked flask, add 5.00 g of apixaban, turn on stirring, add 1.26 g of fumaric acid and 20 mL of purified water to another 50 mL beaker, and stir to dissolve, add the fumaric acid aqueous solution to the above-mentioned there-necked flask, and the system Heated to 65°C, the system was dissolved and then cooled to -10°C ~ 0°C, kept stirring for 1~2 hours, filtered, collected the solid at 60°C-70°C ~ vacuum-dried for 4 hours to obtain 4.97g of white solid, the detected melting point was : 243.0~244.0 ℃, confirmed to be the new crystal form of apixaban, and the purity detected by HPLC was: 99.52%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com