Application of small molecule compound in preparation of urokinase receptor inhibitor medicine
A small molecule compound, urokinase receptor technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
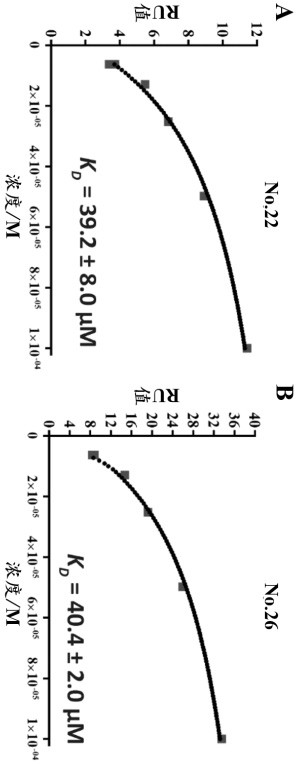
[0025] Example 1: Determination of the binding strength of diltiazem and glyburide to uPAR by surface plasmon resonance (SPR).
[0026] experimental method:
[0027] To determine the equilibrium dissociation constants (K D ), surface plasmon resonance analysis was performed on a BIACORE T200 instrument (BIACORE, Uppsala, Sweden). Coupling of uPAR(H47C / N259C) to the CM5 chip was performed by injecting uPAR(H47C / N259C) protein at a concentration of 30 μg / mL in coupling buffer (10 mM sodium acetate, pH 5.0) to achieve a coupling level of approx. 500 reaction units (RU). Surface blocking with ethanolamine after coupling. A reference channel was prepared in the same way without coupling uPAR (H47C / N259C). Running buffer (20 mM pH7.5 HEPES + 0.15 M NaCl + 0.005% (w / v) containing 100 μM small molecule compound diltiazem or glyburide) was injected at 25 °C at a flow rate of 30 μL per minute. Tween20). Subsequently, dissociation was monitored for 300 seconds. Record the increase...
Embodiment 2
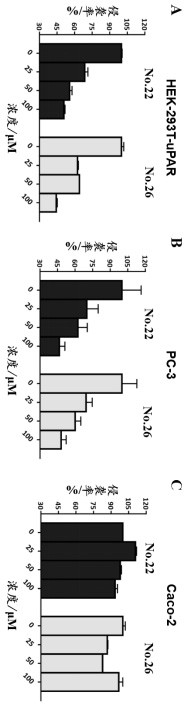
[0030] Example 2: Transwell invasion assay to identify the in vitro anti-tumor metastatic efficacy of diltiazem and glyburide.
[0031] experimental method:
[0032] For migration experiments, place 5 x 10 in serum-free medium 4 Individual cells (HEK-293T-uPAR cells, or PC-3 cells or Caco-2 cells) were placed in the upper chamber of a Transwell permeable scaffold with a cell culture insert with 8 μm pores. PC-3 cells and Caco-2 cells were purchased from Shanghai Institute of Cell Research, Chinese Academy of Sciences. HEK-293T-uPAR cells highly expressed urokinase receptor (uPAR), which was provided by the School of Chemistry, Fuzhou University. For invasion experiments, the preparation of BD Matrigel-coated chambers was done in strict accordance with the operating instructions provided by the Corning supplier. Briefly, 100 μL of a 50-fold dilution of BD Matrigel at a concentration of approximately 0.2–0.3 mg / mL was spread on the upper chamber of a Corning Transwell chamber a...
Embodiment 3
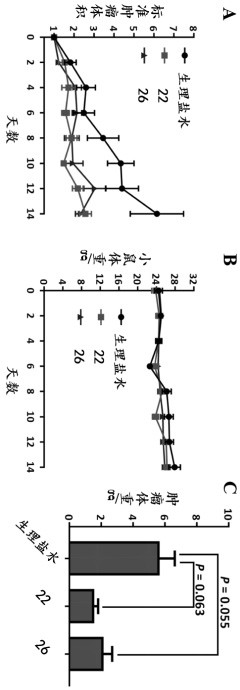
[0035] Example 3: The mouse CT-26 tumor model verifies the anti-tumor effects of diltiazem and glyburide in vivo.
[0036] experimental method:
[0037] will be about 10 7 Viable CT-26 cells (CT-26 cells were purchased from Shanghai Institute of Cell Research, Chinese Academy of Sciences) were suspended in 200 μL of sterile saline and inoculated into the lower right side of the back of each Balb / c mouse. When a palpable tumor was detected, the size of the tumor was monitored daily with a vernier caliper. The volume of the tumor was calculated using the modified ellipsoid formula 1 / 2 × (length × width 2 )computational. When tumor volume reaches 80-100 mm within 3-5 days after inoculation 3 , mice were randomly divided into three groups (5 mice in each group), and the average starting tumor size (80-120 mm) 3 ) and body weight (20~25 grams). Mice were orally administered normal saline, 100 mg / kg diltiazem, or 300 mg / kg glyburide every two days for 14 days. The body weight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com