Polyamino acid hydrogel as well as preparation method and application thereof
A polyamino acid and hydrogel technology, applied in medical science, prosthesis, etc., can solve the problems of few types of monomers, low imbibition, narrow application range, etc., and achieves simple and convenient synthesis method, easy operation and good degradation performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] In a first aspect, the present invention provides a method for preparing a polyamino acid hydrogel, the method comprising the steps of:
[0065] (1) Mix glutamic acid-N-carboxyl internal acid anhydride or glutamic acid-N-carboxyl internal acid anhydride modified with side chain and mercapto-containing N-carboxyl internal acid anhydride with the first solvent, and then add an initiator to carry out polymerization reaction , obtain polyamino acid I, preferably, described polyamino acid I is polyamino acid P(EG 3 Glu-co-Cys); in a specific embodiment of the present invention, preferably, before performing the following step (2), the step of purifying the polyamino acid I is also included; in some preferred embodiments of the present invention In the embodiment of , the glutamic acid-N-carboxyl internal acid anhydride modified by the side chain is a triethylene glycol monomethyl ether modified L -EG 3 GluNCA, the N-carboxyl anhydride containing a sulfhydryl group is cyste...
Embodiment 1
[0102] ① Synthesis of polyamino acid P(EG 3 Glu-co-Cys)
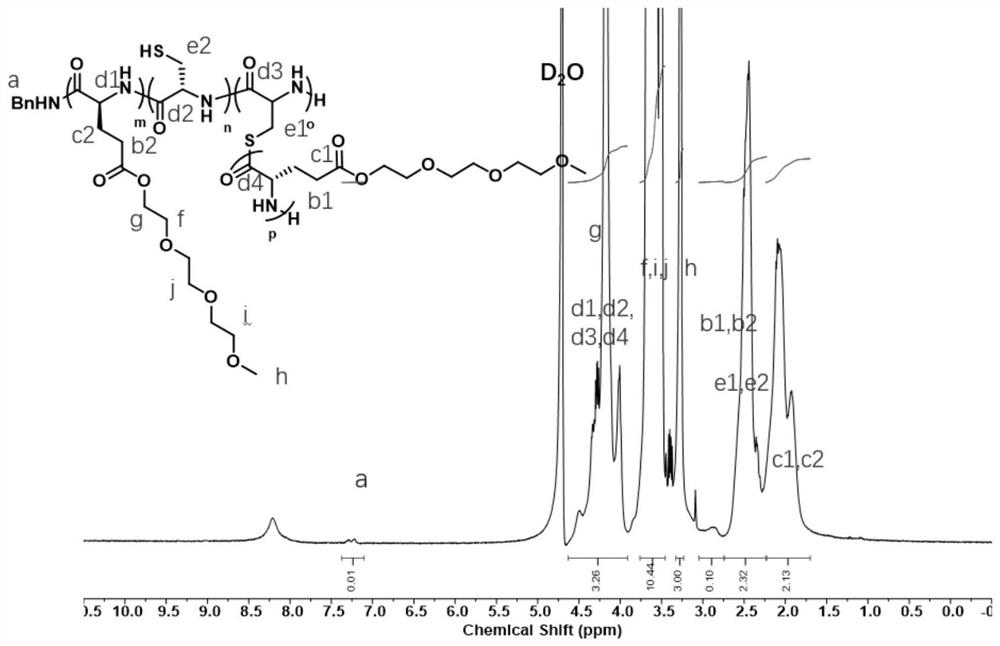
[0103] at room temperature, the L -EG 3 GluNCA (9.3 mmol) with L -CysNCA (1.8 mmol) was dissolved in DMF (3.0 mL), benzylamine initiator (75 μL×0.5 M) was added, and the reaction was stirred at room temperature and 25° C. for 4 h. Complete polymerization was checked using FT-IR. After the polymerization, it was precipitated with ether and centrifuged at 4000 rpm for 10 min. Discard the supernatant. The polymer was further purified by dialysis, and finally a white powdery solid (80% yield) was obtained, which was polyamino acid P(EG 3 Glu-co-Cys). The obtained polyamino acid P(EG 3 Glu-co-Cys) has a branched structure, which is specifically characterized as: the thiol content in the polymer is characterized by Ellman's reagent, and in the obtained results, each segment contains 7.5 thiols; the total amino / thiol content in the polymer is determined by TNBS. Reagent characterization, in the obtained results, each ...
Embodiment 2
[0115] ①Synthesis of polyglutamic acid-polycysteine copolymer P(Glu-co-Cys)
[0116] At room temperature, glutamic acid-N-carboxyl anhydride ( L -GluNCA) (5mmol) with L -CysNCA (1 mmol) was dissolved in DMF (10.0 mL), benzylamine initiator (100 μL×0.5 M) was added, and the reaction was stirred at 10° C. for 18 h. Complete polymerization was checked using FT-IR. After the polymerization, it was precipitated with ether and centrifuged at 4000 rpm for 10 min. Discard the supernatant. The polymer was further purified by dialysis, and finally a white powdery solid (yield 50%) was obtained, which was polyamino acid P(Glu-co-Cys). The obtained polyamino acid P(Glu-co-Cys) has pH-dependent dissolution behavior, dissolves well in a phosphate buffer of pH=7.4, and is insoluble in a weakly acidic environment. Using this property, smart gels with responsive pore / mechanical properties can be designed; the reaction formula for the synthesis of polyglutamic acid-polycysteine copolym...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com