PH-responsive siRNA delivery system
A delivery system and responsive technology, applied in the direction of organic active ingredients, medical formulations with non-active ingredients, and medical formulations containing active ingredients, etc., can solve the problem of prolonged siRNA circulation time, low tumor site concentration, and poor biodistribution and other problems, to achieve the effect of enhancing target cell uptake, process stability, and inhibiting non-specific adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
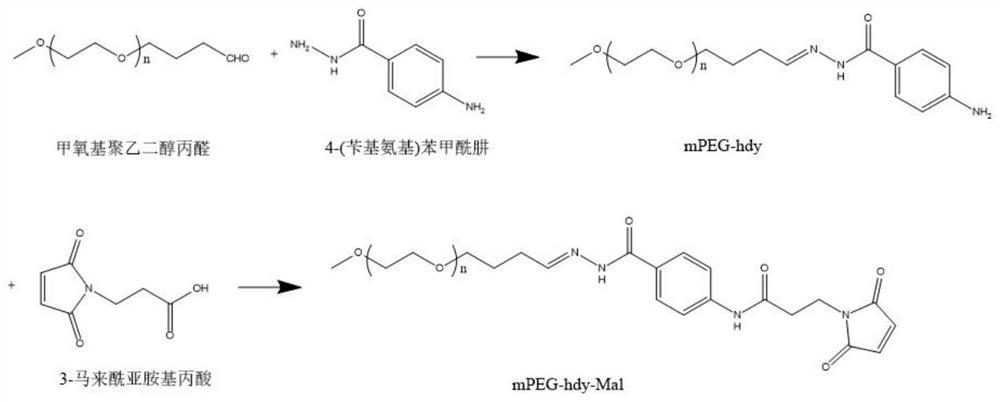
[0049] The preparation of embodiment 1mPEG-hdy-Mal
[0050] The synthetic reaction path of mPEG-hdy-Mal is as follows figure 1 As shown, the specific: the first step: the methoxy polyethylene glycol propionaldehyde (mPEG-CHO) and 4-(benzylamino) benzoic hydrazide according to the molar ratio of 1:2 in anhydrous ethanol system Stir, add an equivalent of acetic acid as a catalyst; reflux the reaction mixture at 80°C for 6-8 hours under nitrogen protection, cool the reaction mixture to room temperature, collect the precipitate by filtration, and remove residual residues by simple washing with dichloromethane Starting materials and impurities yielded a PEG derivative (mPEG-hdy) containing a hydrazone bond (hdy). In the second step, mPEG-hdy and 3-maleimidopropionic acid obtained above were added to the dichloromethane solution at a molar ratio of 1:5, and the mixture was stirred for 4 hours. The mixture was washed with saturated ammonium chloride solution and then twice with sat...
Embodiment 2
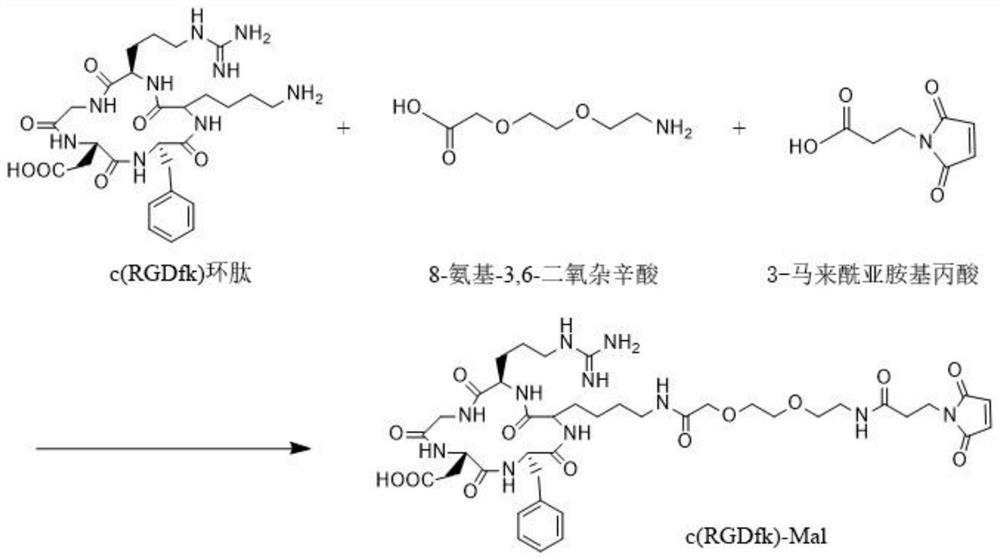
[0051] Example 2 Preparation of c(RGDfk)-Mal
[0052] c(RGDfk) polypeptide (12.1 mg) and 8-amino-3,6-dioxaoctanoic acid (3.02 mg) were stirred in polyethylene glycol solution under nitrogen at room temperature for 4-8 h until all starting peptides were After consumption, 3-maleimidopropionic acid (6.76 mg) was added to continue stirring the reaction for 4-8 h. The resulting crude product was purified by RP-MPLC. (Chromatographic conditions: injection volume 50 μL; column temperature 40 °C; flow rate 2.5 mL / min; mobile phase: phase A 0.1% TFA acetonitrile, phase B 0.1% water; elution program: 0~30 min, 5%~50% A ; 30~32min, 50%~5%A).
[0053] The obtained c(RGDfk)-Mal was characterized by mass spectrometry (UHPLC-Q Exactive Orbitrap quadrupole combined electrostatic orbitrap LC / MS was used for detection, electrospray ion source (H-ESI) was used, and the sheath gas flow rate was 30L / min, curtain gas flow rate 10L / min, nozzle voltage +4000V, capillary temperature 320ºC, curtai...
Embodiment 3c
[0054] Example 3c Synthesis of (RGDfk)-PEG-siRNA
[0055] The synthetic route of c(RGDfk)-PEG-siRNA is as follows Image 6 shown. Specifically: The intermediates and mPEG-hdy-Mal and c(RGDfk)-Mal were successfully synthesized by the above-mentioned Example 1 and Example 2, and the sulfhydryl (-SH) modified nucleic acid single chain was constructed by conventional nucleic acid chemical synthesis method. Monomer modification at the 5' end of the nucleic acid chain: After phosphorylation of the hydroxyl group at the 5' position of the pentose sugar in the nucleotide unit at the 5' end of the siRNA sense strand, a sulfhydryl (-SH) group is attached. Monomer modification at the 3' end of the nucleic acid strand: After phosphorylation of the hydroxyl group at the 5-position of the pentose sugar in the nucleotide unit at the 3' end of the siRNA antisense strand, a sulfhydryl (-SH) group is attached. Synthesize single-stranded sense strand ssRNA and antisense strand asRNA according ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com