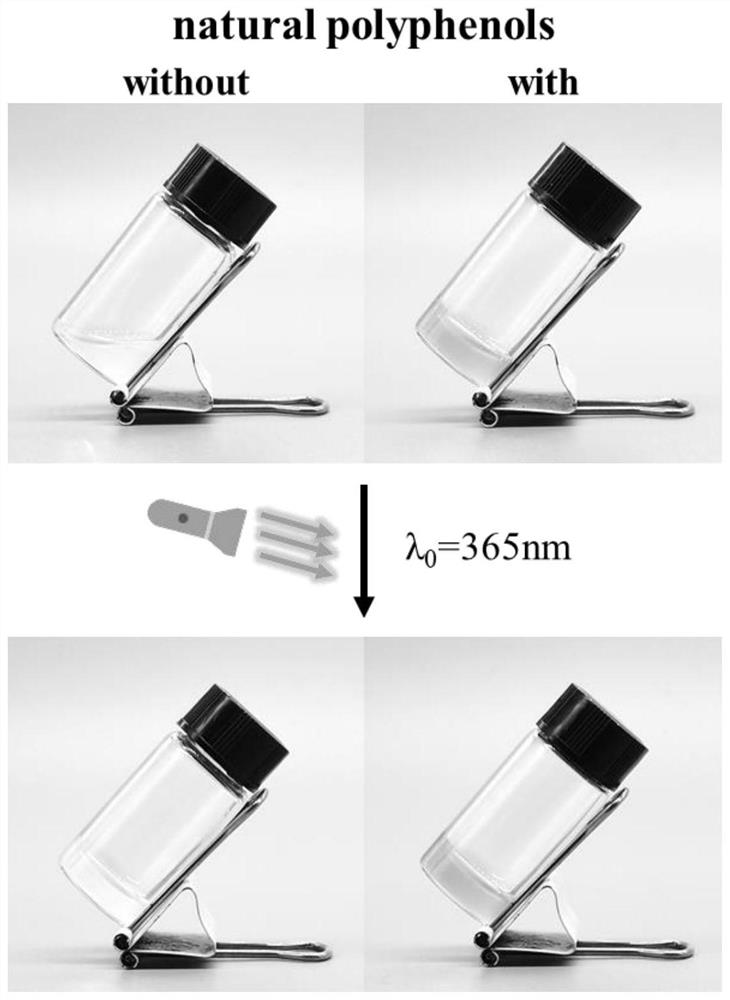
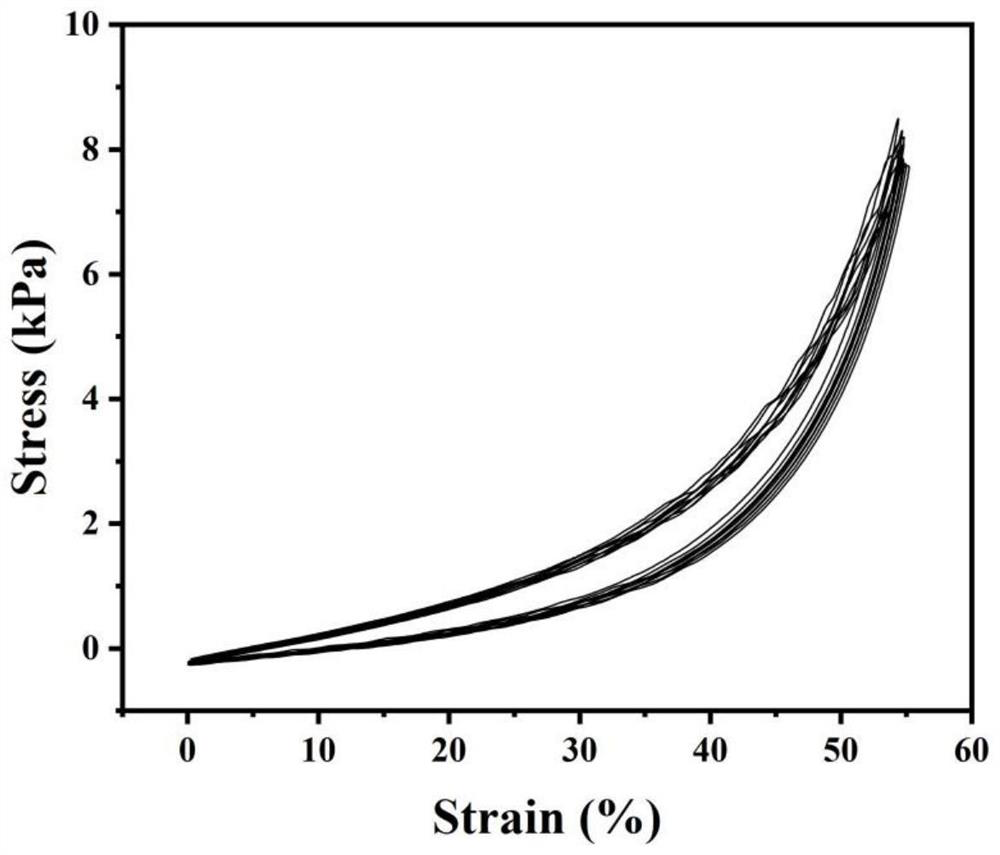
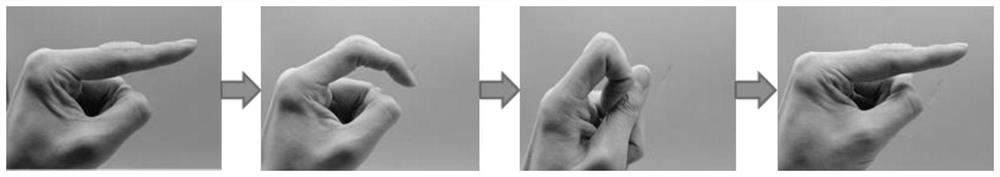
Preparation method of injectable bi-crosslinking hydrogel dynamically combined with natural polyphenol
A technology of cross-linked hydrogel and natural polyphenols, which is applied in the fields of medical science and prostheses, can solve the problems of easy leakage of precursor liquid to surrounding tissues, insufficient mechanical properties, poor tissue adhesion and easy falling off, and achieve good anti-aging Effects of angiogenesis, enhanced mechanical properties, excellent water retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Dissolve 1 g of hyaluronic acid with a molecular weight of 100 to 150 W in 300 mL of deionized water, stir and dissolve fully, add 1.48 mL of methacrylic anhydride under ice bath conditions, and then maintain the pH of the solution by continuously adding 1M sodium hydroxide. 8, and then continued to react under ice bath conditions for 24 hours. After the reaction, the reaction product was placed in a dialysis bag with a molecular weight cut-off of 8000-14000 and dialyzed with deionized water for 3 days, and the water was changed 3 times a day.
[0033] 2) Dissolve 1g of HAMA in 300mL of 0.1M morpholine ethanesulfonic acid buffer solution, fully stir to dissolve, add 1.46g of DMTMM to activate the carboxyl group on the hyaluronic acid molecular chain, and add 0.272g of 3-aminophenylboronic acid after half an hour, The reaction was carried out for 48 hours. After the reaction, the reaction product was placed in a dialysis bag with a molecular weight cut-off of 8000-14...
Embodiment 2
[0041] 1) Dissolve 1 g of hyaluronic acid with a molecular weight of 100-150 W in 300 mL of deionized water, stir to dissolve, add 2.96 mL of methacrylic anhydride under ice-bath conditions, and maintain the pH of the solution by continuously adding 1M sodium hydroxide. 8, and then continued to react under ice bath conditions for 24 hours. After the reaction, the reaction product was placed in a dialysis bag with a molecular weight cut-off of 8000-14000 and dialyzed with deionized water for 3 days, and the water was changed 3 times a day.
[0042] 2) Dissolve 1g of HAMA in 300mL of 0.1M morpholine ethanesulfonic acid buffer solution, stir to dissolve, add 0.73g of DMTMM to activate the carboxyl group on the hyaluronic acid molecular chain, add 0.136g of 3-aminophenylboronic acid after half an hour, React for 24 hours. After the reaction, the reaction product was placed in a dialysis bag with a molecular weight cut-off of 8000-14000 and dialyzed with deionized water for 3 days...
Embodiment 3
[0049] 1) Dissolve 1 g of hyaluronic acid with a molecular weight of 100-150 W in 300 mL of deionized water, stir to dissolve, add 2.22 mL of methacrylic anhydride under ice bath conditions, and maintain the pH of the solution to 1 M sodium hydroxide. 8, and then continued to react under ice bath conditions for 12 hours. After the reaction, the reaction product was placed in a dialysis bag with a molecular weight cut-off of 8000-14000 and dialyzed with deionized water for 3 days, and the water was changed 3 times a day.
[0050] 2) Dissolve 1g of HAMA in 300mL of 0.1M morpholine ethanesulfonic acid buffer solution, stir to dissolve, add 1.095g of DMTMM to activate the carboxyl group on the molecular chain of hyaluronic acid, add 0.068g of 3-aminophenylboronic acid after half an hour, React for 24 hours. After the reaction, the reaction product was placed in a dialysis bag with a molecular weight cut-off of 8000-14000 and dialyzed with deionized water for 3 days, and the water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com