Meso-2, 3-butanediol dehydrogenase as well as mutant and application thereof
A technology of meso-2 and butanediol dehydrogenase, which is applied in the field of enzyme engineering, can solve the problems of incompatibility between stability and activity, and achieve the effects of high stability, high temperature resistance and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
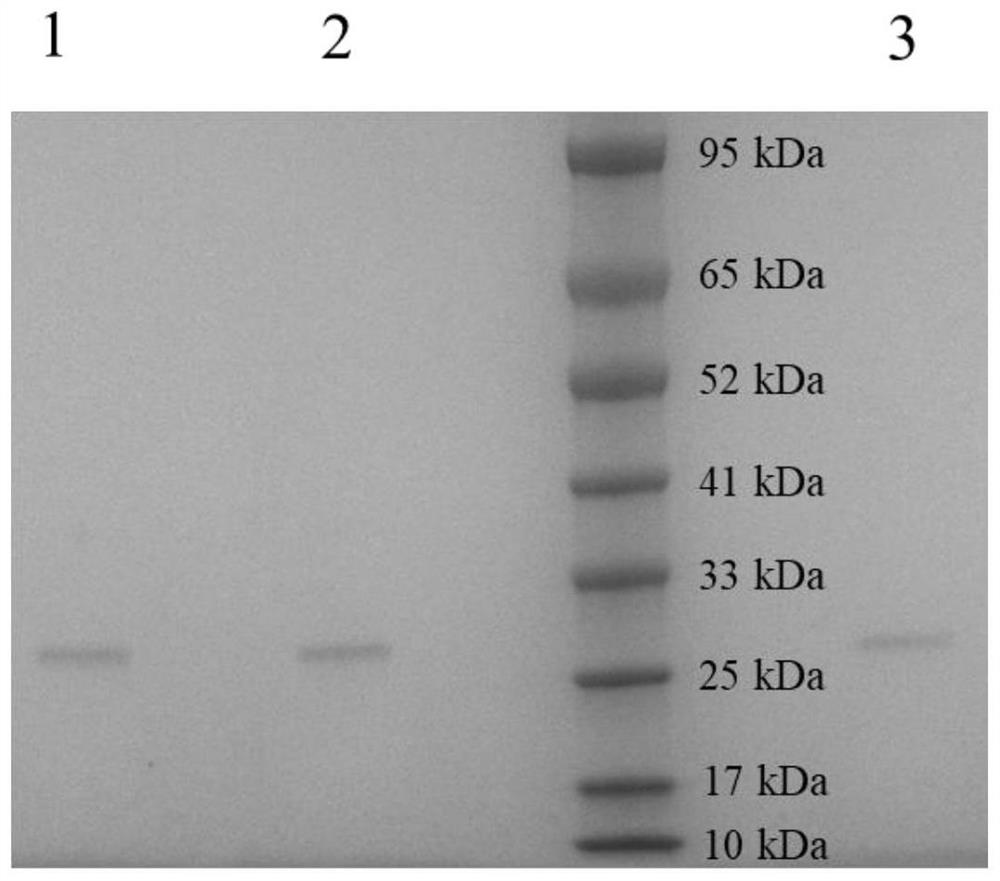
[0047] Example 1 Cloning, expression, purification and temperature tolerance determination of meso-2,3-butanediol dehydrogenase
[0048] 1. Cloning of meso-2,3-butanediol dehydrogenase (LlBDH for short)
[0049] The meso-2,3-butanediol dehydrogenase gene was cloned from Lactococcus lactis, inserted into pET28a-SUMO plasmid, and then transformed into E. coli BL21 (DE3) to obtain pET28a -SU MO-LlBDH expressing strain.
[0050] The specific steps are:
[0051] 1. Extraction of Lactococcus lactis genome
[0052] Lactococcus lactis genome was extracted using TaKaRa MiniBEST Bacteria Genomic DNA Extraction Kit Ver.3.0, as follows:
[0053] ①Collect the cultured Lactococcus lactis bacterial liquid with a 1.5mL centrifuge tube, centrifuge at 12000rpm for 2min, and discard the supernatant;
[0054] ②Add 180μL of Buffer GL, 20μL of Proteinase K (20mg / mL) and 10μL of RNase A (10mg / mL), shake and mix well, and incubate in a water bath at 56°C for 10min. At this time, the solution shou...
Embodiment 2
[0108] Example 2 Design of mutation sites based on product release process
[0109] Gaussian accelerated molecular dynamics (GaMD) is an unconstrained enhanced sampling method. The repeated dissociation and binding of the captured small molecule ligand to the enzyme is simulated using LiGaMD on the nanosecond time scale. Molecular dynamics simulations were performed using Amber20 software. protein using the ff19SB force field, NAD + and NADH cofactor using the force field constructed by Holmberg et al. Add explicit OPC water molecules, the protein is from the edge of the box Electroneutralization was then performed with an ionic concentration of 0.15M NaCl. Energy minimization is divided into three stages. The first stage only minimizes the positions of solvent molecules and ions; the second stage minimizes hydrogen atoms; the third stage unconstrained minimizes all atoms in the simulated system. Each stage of minimization consists of 2500 steepest descent steps and 250...
Embodiment 3
[0111] Example 3 Construction of single-point saturation mutation
[0112] A single point saturation mutation was performed on LlBDH. The specific method is as follows:
[0113] 1. Whole plasmid PCR
[0114] Taking the pET28a-SUMO-L1BDH plasmid as a template, the upstream and downstream primers (Table 1) covering the mutation point were designed to carry out whole plasmid PCR:
[0115] Table 2 Primers used for construction of single point mutation library
[0116]
[0117] PCR amplification system:
[0118]
[0119] PCR amplification conditions:
[0120] 1) Pre-denaturation: 95℃ for 5min;
[0121] 2) Denaturation: 98°C for 10s; annealing: 60°C for 15s; extension: 72°C for 10s; a total of 30 cycles;
[0122] 3) Post extension: 72°C for 10min;
[0123] 4) Store at 4°C.
[0124] 2. Template digestion:
[0125] The PCR product was subjected to agarose gel electrophoresis, and after recovery, the plasmid template digested with DpnI enzyme was digested as follows: Dpn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com