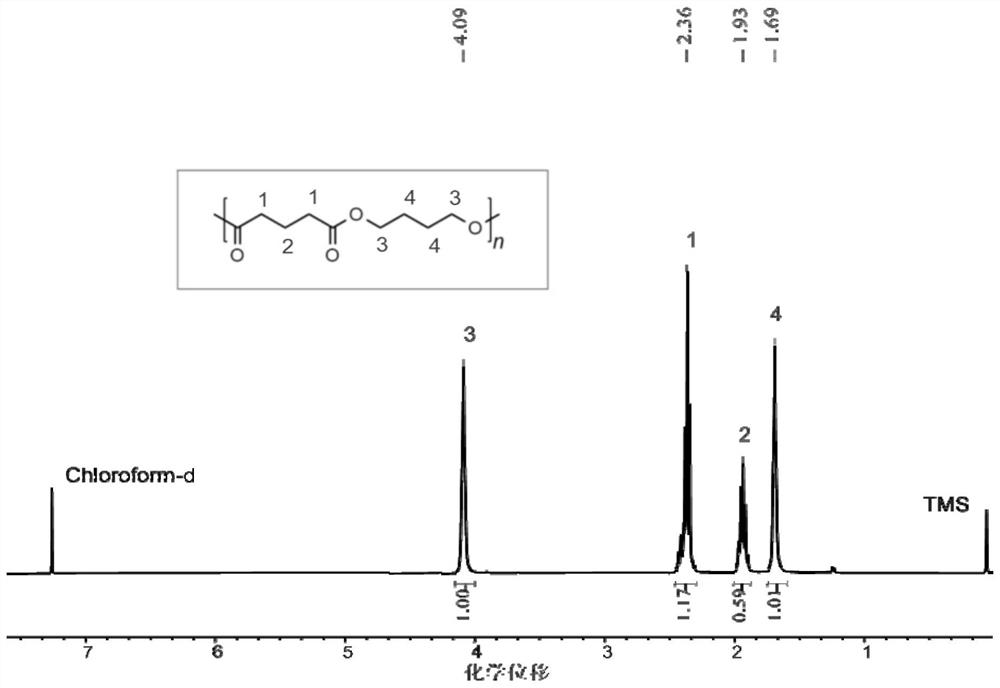
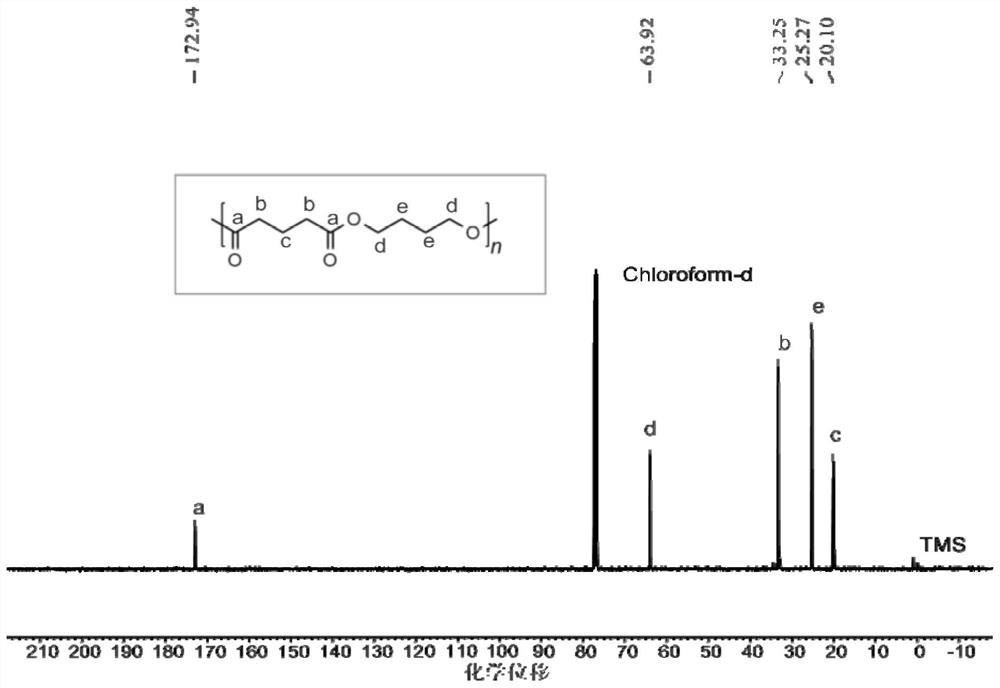
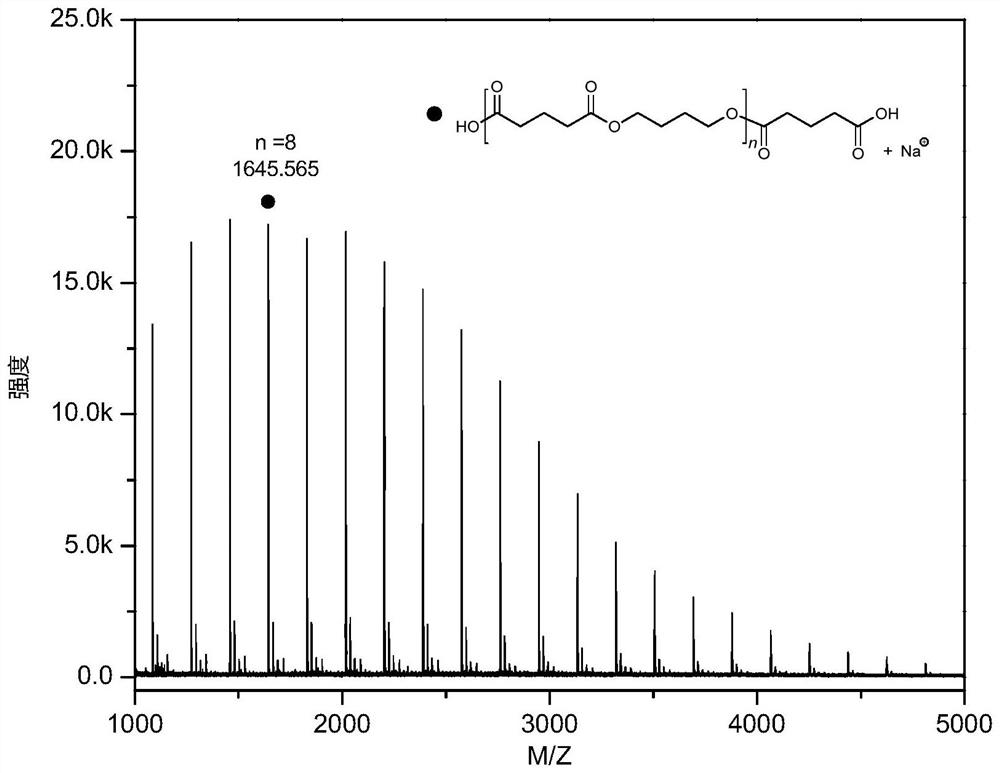
Method for preparing polyester material by depolymerizing polytetrahydrofuran-based material and product thereof
A technology of polytetrahydrofuranyl and tetrahydrofuranyl, which is applied in the field of depolymerization and recovery of polymer materials, and achieves the effects of low energy consumption, mild process conditions and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Polytetrahydrofuran (PTHF-650) / glutaric anhydride with a molecular weight of 650 was alternately copolymerized into polyester
[0050] Before the polymerization reaction, remove moisture from a 10 mL Shrek tube at 110°C for about 2 hours and cool it to room temperature in a desiccator; add a certain mass of CF to the Shrek tube in turn. 3 SO 3 H, polytetrahydrofuran with a number-average molecular weight of 650 Da, glutaric anhydride. CF 3 SO 3 The molar ratio of a single polytetrahydrofuran segment / glutaric anhydride in H / polytetrahydrofuran having a molecular weight of 650 is 3 / 200 / 300. Placed at 100 ° C under autogenous pressure for 4 h. After the reaction, the crude product was first dissolved in dichloromethane, and then the polymer was precipitated in 100 mL of methanol / hydrochloric acid mixture (hydrochloric acid molar concentration was 5%), repeated washing three times, and vacuum drying to constant weight. The molecular weight and molecular weigh...
Embodiment 2
[0052] Example 2 Polytetrahydrofuran (PTHF-650) / glutaric anhydride with a molecular weight of 650 was alternately copolymerized into polyester
[0053] Before the polymerization reaction, remove moisture from a 10 mL Shrek tube at 110°C for about 2 hours and cool it to room temperature in a desiccator; add a certain mass of BF to the Shrek tube in turn. 3 , the molecular weight of 650 polytetrahydrofuran, glutaric anhydride. BF 3 The molar ratio of a single polytetrahydrofuran chain segment / glutaric anhydride in polytetrahydrofuran with a molecular weight of 650 is 3 / 200 / 300. Placed at 100 ° C under autogenous pressure for 4 h. After the reaction, the crude product was first dissolved in dichloromethane, and then the polymer was precipitated in 100 mL of methanol / hydrochloric acid mixture (hydrochloric acid molar concentration was 5%), repeated washing three times, and vacuum drying to constant weight. The molecular weight and molecular weight distribution of the polymer we...
Embodiment 3
[0054] Example 3 Polytetrahydrofuran (PTHF-650) / glutaric anhydride with a molecular weight of 650 was alternately copolymerized into polyester
[0055] Before the polymerization reaction, a 10 mL Shrek tube was removed at 110°C for about 2 hours to remove moisture and cooled to room temperature in a desiccator; a certain mass of InBr was added to the Shrek tube in turn. 3 , the molecular weight of 650 polytetrahydrofuran, glutaric anhydride. InBr 3 The molar ratio of a single polytetrahydrofuran chain segment / glutaric anhydride in polytetrahydrofuran with a molecular weight of 650 is 3 / 200 / 300. Placed at 100 ° C under autogenous pressure for 4 h. After the reaction, the crude product was first dissolved in dichloromethane, and then the polymer was precipitated in 100 mL of methanol / hydrochloric acid mixture (hydrochloric acid molar concentration was 5%), repeated washing three times, and vacuum drying to constant weight. The molecular weight and molecular weight distributio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com