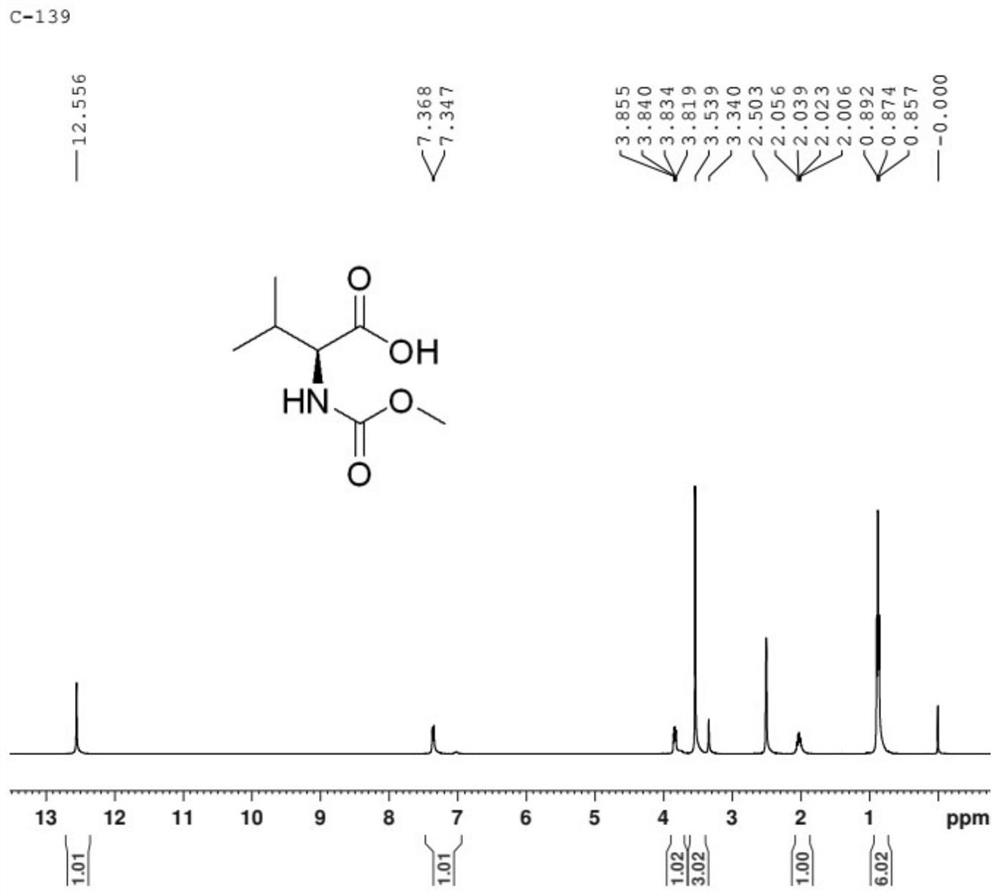
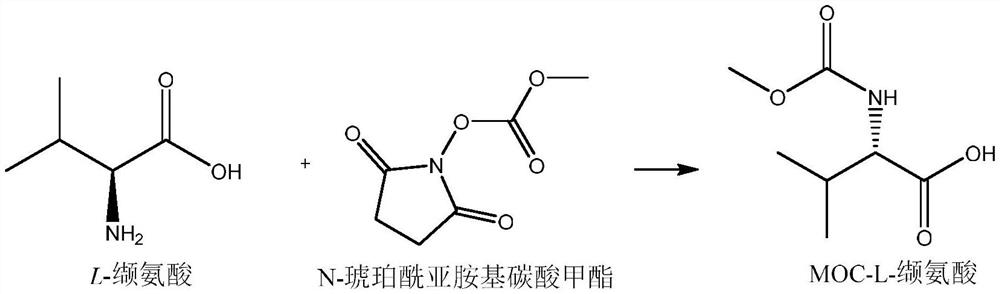
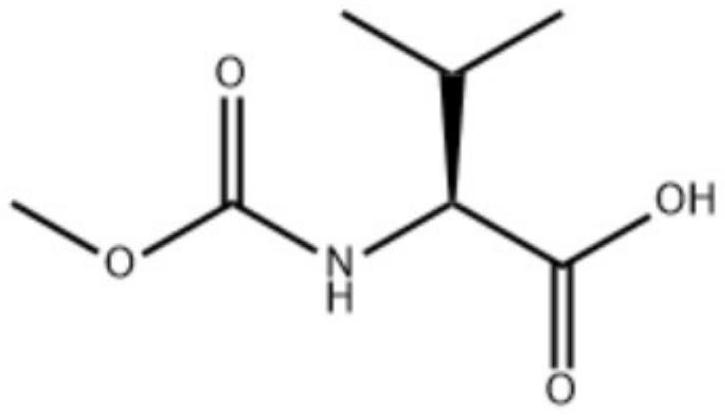
Synthesis process of MOC-L-valine
A technology of MOC-L-, synthesis process, applied in the field of MOC-L-valine synthesis process, can solve the problem of irritating odor and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Put 110g of water and 40g of liquid caustic soda with a mass fraction of 32% into a 500ml four-necked bottle, turn on stirring, control the temperature of the kettle to be no higher than 20°C, put in 35g of L-valine, and stir until all the solids are dissolved. The internal temperature of the reaction kettle was controlled to be 5-20°C, and 56.9 g of N-succinimidyl methyl carbonate was added in batches at the same time. After the feeding was completed, the internal temperature of the reaction kettle was controlled to be 50-60°C, and the temperature was maintained and stirred for 2-3 hours. Sampling in the control, requires L-valine ≤ 2%. Add 160 g of toluene to the reaction solution, cool down to below 15°C, dropwise add sulfuric acid to adjust pH=2~3, a large amount of solid salt does not dissolve, be warming up to 23~28°C and stir the salt to dissolve completely, separate the water layer to obtain the organic layer. The organic layer was washed three times with 15% br...
Embodiment 2
[0037] Put 110g of water and 53g of sodium carbonate into a 500ml four-necked bottle, turn on stirring, control the temperature of the kettle to be no higher than 20°C, put in 35g of L-valine, and stir until all the solids are dissolved. The internal temperature of the reaction kettle was controlled to be 5-20°C, and 56.9 g of N-succinimidyl methyl carbonate was added in batches at the same time. After the feeding was completed, the internal temperature of the reaction kettle was controlled to be 10-20°C, and the temperature was kept and stirred for 2-3 hours. Sampling in the control, requires L-valine ≤ 2%. Add 160 g of ethyl acetate to the reaction solution, cool down to below 15°C, dropwise add sulfuric acid to adjust pH=2~3, a large amount of solid salt does not dissolve, be warming up to 23~28°C and stir the salt to dissolve completely, separate the water layer to obtain the organic layer . The organic layer was washed with 15% brine, and the internal temperature was con...
Embodiment 3
[0039]Put water 110g and sodium bicarbonate 84g into a 500ml four-neck flask, turn on stirring, control the temperature of the kettle to be no higher than 20°C, put in 35g of L-valine, and stir until all the solids are dissolved. The internal temperature of the reaction kettle was controlled to be 5-20°C, and 56.9 g of N-succinimidyl methyl carbonate was added in batches at the same time. After the feeding was completed, the internal temperature of the reaction kettle was controlled to be 10-20°C, and the temperature was kept and stirred for 2-3 hours. Sampling in the control, requires L-valine ≤ 2%. Add 160 g of tetrahydrofuran to the reaction solution, cool down to below 15°C, dropwise add sulfuric acid to adjust pH=2~3, a large amount of solid salt does not dissolve, heat up to 23~28°C and stir the salt to dissolve completely, separate the water layer to obtain the organic layer. The organic layer was washed with 15% brine, and the internal temperature was controlled not to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com