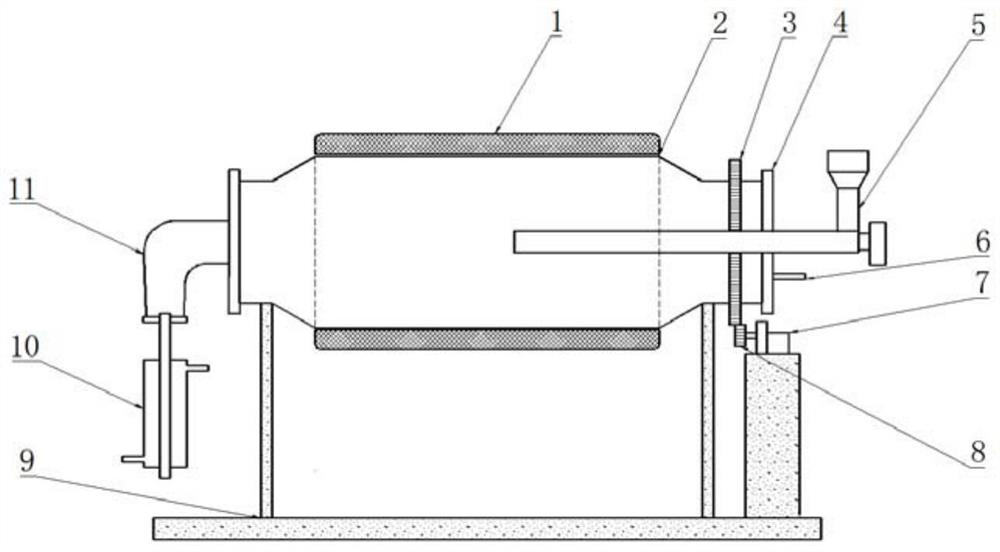
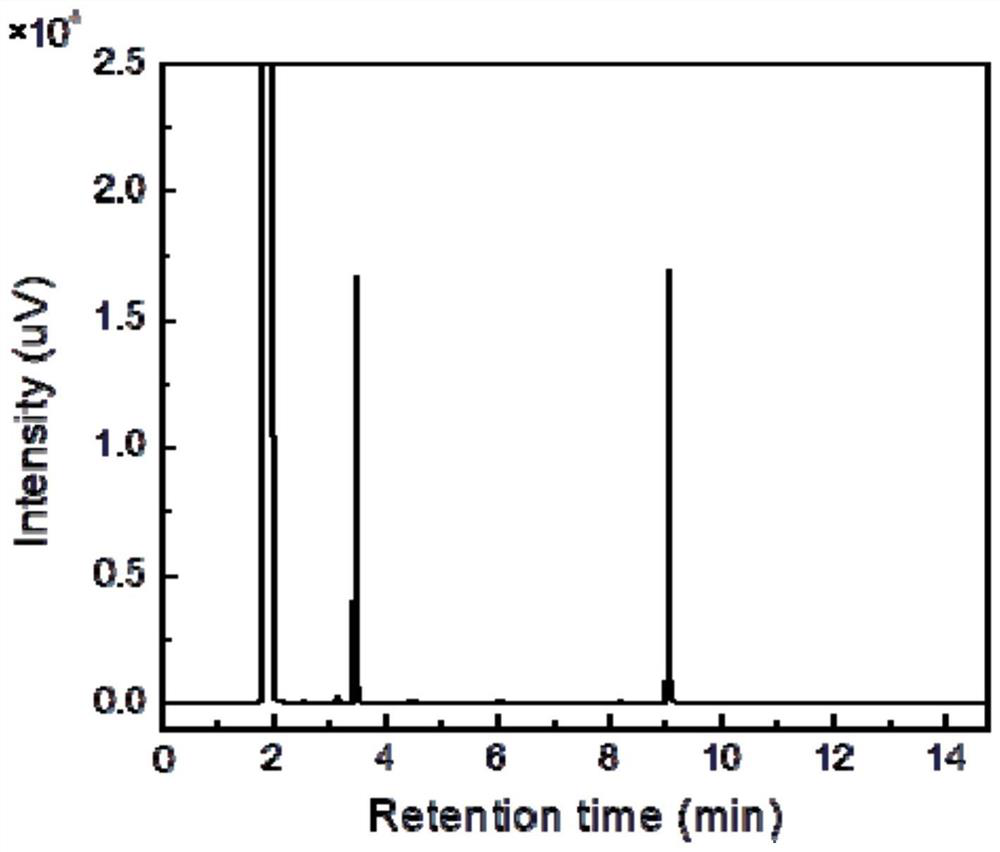
Method and reaction device for catalytically depolymerizing polymethyl methacrylate into monomer
The technology of polymethyl methacrylate and reaction device is applied in chemical instruments and methods, preparation of carboxylate, preparation of organic compounds, etc., and can solve the problems of fluidized bed bed collapse, serious coking, high viscosity, etc. Achieve the effect of increasing dispersibility and mixing uniformity, reducing pyrolysis temperature and avoiding energy loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A method for catalytic depolymerization of polymethyl methacrylate into monomer, comprising the steps:
[0041] S1: Prepare the first catalyst, which is a spherical Al with a diameter of 3 mm without metal oxide support 2 O 3 Particles with a specific surface area of 360m 2 / g.
[0042] S2: Weigh 1500g of spherical Al in step S1 2 O 3 , added to the rotary furnace reactor, in N 2 The rotary furnace reactor was heated to a depolymerization temperature of 400°C under atmosphere (20ml / min).
[0043] S3: 150g of discarded polymethyl methacrylate is added to the rotary furnace reactor of step S2 by the screw feeder, the feed rate is 5g / min, and the thermal depolymerization reaction occurs under normal pressure for 30min, and the monomer gas is removed from the rotary furnace. The outlet of the reactor was discharged, and the liquid phase product was collected after condensation, and finally 134.6 g of liquid oil was obtained.
[0044] The rotary furnace reactor of t...
Embodiment 2
[0049] A method for catalytic depolymerization of polymethyl methacrylate into monomer, comprising the steps:
[0050] S1: Prepare the first catalyst, which is a spherical Al with a diameter of 3 mm supported on MgO and NiO 2 O 3 (The mass ratio of MgO and NiO is 1:1, and the loading amount is 3 wt%). First, the metal oxide catalyst was prepared by an equal volume ultrasonic impregnation method. 11.0 g of magnesium nitrate and 11.7 g of nickel nitrate hexahydrate were dissolved in 240 g of deionized water, and the aqueous solution was added to a spherical Al containing 3 mm in diameter. 2 O 3 (194.0g, the specific surface area is 360m 2 / g), ultrasonically vibrated for 5 h to obtain a catalyst precursor. The precursor was dried in a vacuum drying oven at 70 °C for 12 h, then transferred to a tube furnace reactor, calcined in an air atmosphere, and the tube furnace reactor was heated to 650 °C, and at this temperature After holding for 3h, 200g of bimetallic oxide catalyst...
Embodiment 3
[0056] A method for catalytic depolymerization of polymethyl methacrylate into monomer, comprising the steps:
[0057] S1: Prepare the first catalyst, which is a 4mm diameter spherical Al supporting MgO and NiO 2 O 3 (The mass ratio of MgO and NiO is 1:1, the loading is 3wt%), and its specific surface area is 360m 2 / g.
[0058] S2: Weigh 1500g of spherical Al with a diameter of 4 mm supporting MgO and NiO in step S1 2 O 3 , added to the rotary furnace reactor, in N 2 The rotary furnace reactor was heated to a depolymerization temperature of 350°C under atmosphere (20ml / min).
[0059] S3: 150g of waste polymethyl methacrylate is added to the rotary furnace reactor of step S2 by the screw feeder, the feed rate is 5g / min, the thermal depolymerization reaction occurs under normal pressure for 30min, and the monomer gas is reacted from the rotary furnace The outlet of the device was discharged, and the liquid phase product was collected after condensation, and finally 136.8 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com