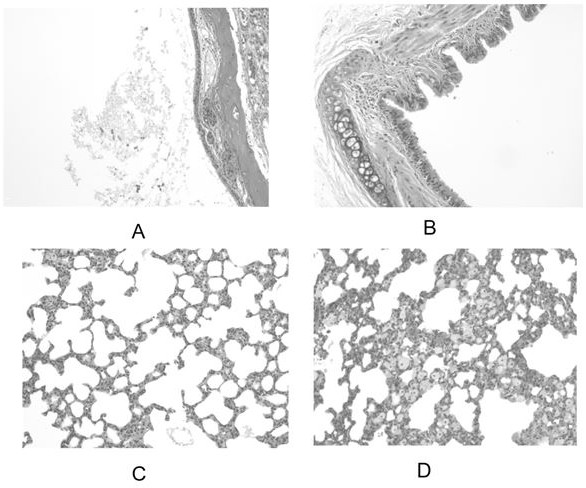
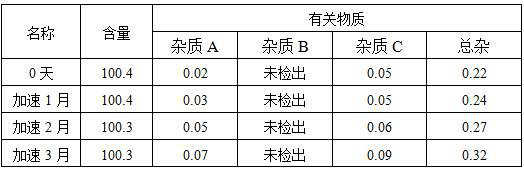
Voriconazole aerosol inhalant and application thereof
A voriconazole and inhalation technology, applied in the field of antifungal drugs, can solve problems such as instability of voriconazole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
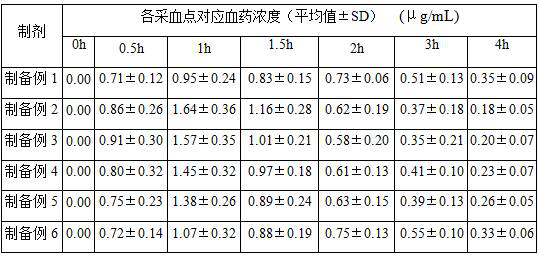
preparation example 1
[0030] (S1) dissolve 0.04 part of polyoxyethylene hydrogenated castor oil and 0.01 part of pentaerythritol monostearate in 15 parts of acetic acid aqueous solution with pH 3, stir evenly, slowly add 1 part of voriconazole original drug, continue to stir until voriconazole is completely dissolved . The obtained acetic acid solution of voriconazole was added to a saturated aqueous solution containing 10 parts of sodium sulfobutyl-β-cyclodextrin, stirred at 30 ° C for 4 h, the inclusion was complete, and the pH was adjusted to 6.5 with 6.5 wt % aqueous sodium carbonate solution , adding 0.1 wt % of activated carbon in the solution, stirring for 0.5 h, and filtering with a 0.22 μm microporous membrane to obtain a clear and transparent solution, which is used for the next step of freeze-drying.
[0031] (S2) Pre-freeze the mixture of the inclusion compounds obtained in (S1) at -30°C for 5h, vacuumize, heat up to -10°C at 5Pa, keep the temperature for 30h, and then slowly heat up to...
preparation example 2
[0038] Other conditions and operations are the same as in Preparation Example 1, except that the solubilizer in step (S1) is 0.05 part of polyoxyethylene hydrogenated castor oil, that is, no pentaerythritol monostearate is added. After testing, the inclusion rate of the voriconazole-cyclodextrin inclusion complex freeze-dried powder obtained in Preparation Example 2 was 96.4%, and in a phosphate buffer solution with pH=7 and a saturated solution at 25°C, the dissolved concentration of voriconazole reached 18.6 mg / mL.
preparation example 3
[0040] Other conditions and operations are the same as in Preparation Example 1, except that the solubilizer in step (S1) is 0.05 part of pentaerythritol monostearate, that is, no polyoxyethylene hydrogenated castor oil is added. After testing, the inclusion rate of the voriconazole-cyclodextrin inclusion complex freeze-dried powder obtained in Preparation Example 3 was 93.2%, and in a phosphate buffer solution with pH=7 and a saturated solution at 25°C, the dissolved concentration of voriconazole reached 18.0 mg / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com