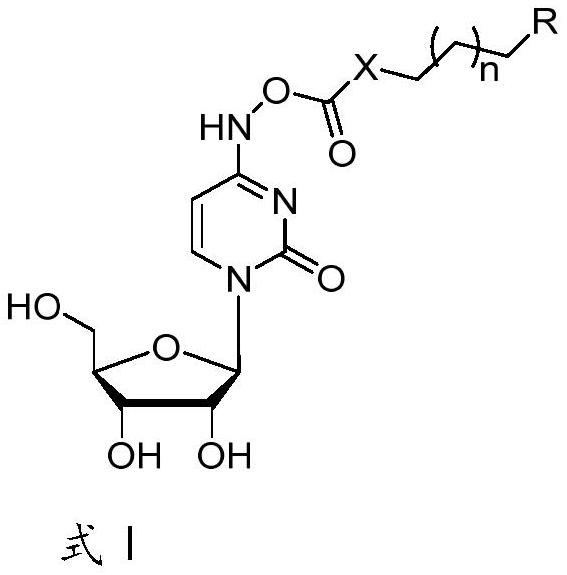
N4-hydroxycytidine lipid prodrug as well as preparation method and application thereof
A pharmaceutical preparation and drug technology are applied in the field of N4-hydroxycytidine lipid prodrug and its preparation, which can solve the problems of poor oral bioavailability and unfavorable absorption, and achieve good application prospects and high anti-new coronavirus activity. , the effect of short preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
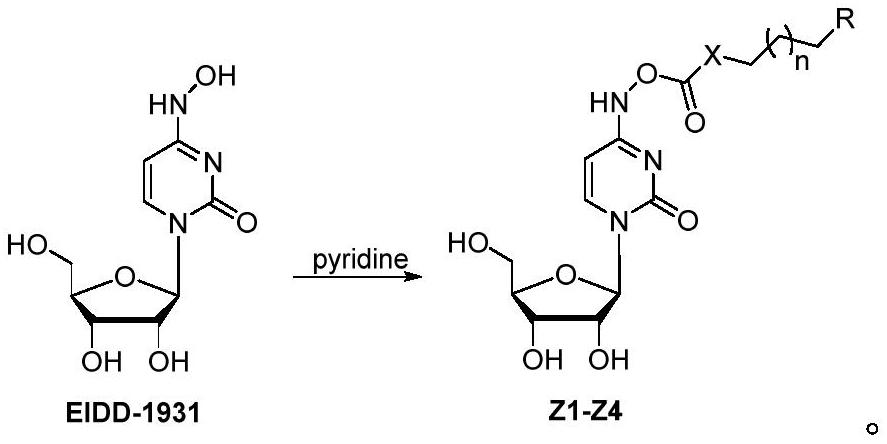
[0040] The preparation method of the compounds Z1-Z4 is as follows: adding acid chloride to the organic reagent solution of the compound EIDD-1931, stirring overnight, and purifying to obtain the compounds Z1-Z4.
[0041] The above reaction process can be carried out at room temperature, and the purification includes the steps of concentration and chromatography.
[0042] Further, the compound EIDD-1931 was dissolved in pyridine.
[0043] Further, the molar ratio of compound EIDD-1931 to acid chloride is 1:1.0-3.0, and further, the molar ratio is 1:1.0-1.5.
[0044] A third aspect of the present invention provides a pharmaceutical composition comprising the compound of the first aspect. More specifically, it acts as an active ingredient. In addition, in addition to the compound described in the first aspect, it may also include other ingredients with antiviral effects, which will not be specifically limited here.
[0045] And, a pharmaceutical preparation, which comprises th...
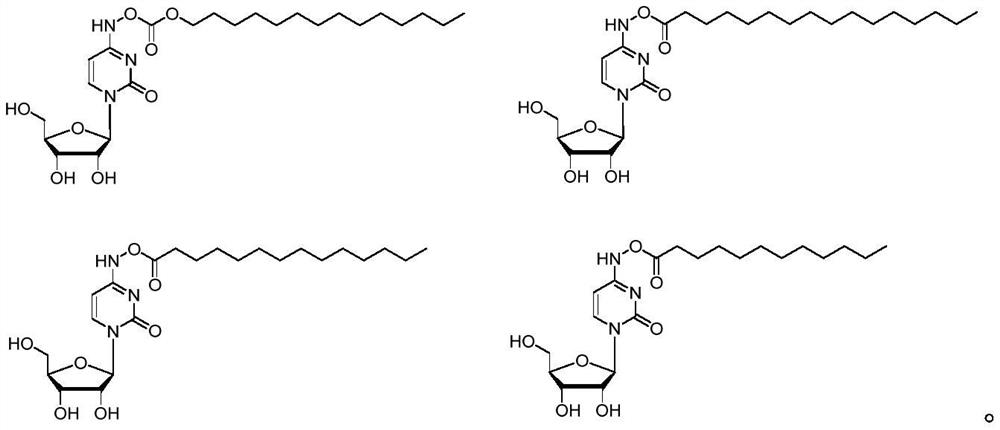
Embodiment 1
[0059] Synthesis of the compound of Example 1
[0060] Prepared according to the following synthetic route
[0061]
[0062] (1) Synthesis of compound Z1
[0063]
[0064] EIDD-1931 (200 mg, 0.77 mmol) was dissolved in pyridine (4 mL), cooled in an ice-water bath, dodecyl chloroformate (0.23 mL, 0.85 mmol) was slowly added dropwise, and the mixture was stirred at room temperature overnight. The solution was evaporated to dryness under reduced pressure, and 300 mg of white solid was obtained by silica gel column chromatography (DCM:MeOH=20:1, 10:1), which was compound Z1, and the yield was 77%. 1 H NMR (400MHz, DMSO-d 6 )δ10.81(s, 1H), 7.44(d, J=8.2Hz, 1H), 5.95(d, J=8.2Hz, 1H), 5.75(d, J=5.9Hz, 1H), 5.32(d, J=4.5Hz, 1H), 5.10–5.00 (m, 2H), 4.12 (t, J=6.6Hz, 2H), 4.02–3.91 (m, 2H), 3.81 (q, J=3.2Hz, 2H), 3.62-3.49(m,2H),1.66-1.55(m,2H),1.37-1.17(m,22H),0.85(t,J=6.6Hz,3H).HRMS calcd for C 24 H 41 N 3 O 8 [M+H] + 500.2966, found 500.2970.
[0065] (2) Synthesis o...
Embodiment 2
[0074] Example 2 In vitro antiviral activity test and cytotoxicity of the compound against SARS-CoV-2
[0075] Vero cells (ATCC-CCL81) at 1 × 10 per well 4 The density of individual cells was seeded in 96-well plates in DMEM 10% FCS medium. After 24 hours of growth, the culture medium was removed, and cells were treated with different concentrations of compounds, using about 100 TCID 50 Mock infection or infection with SARS-CoV-2 per well (final volume 200 μL / well of DMEM 2% FCS). On the 5th day after infection, the viral CPE was recorded under the microscope, and the 50% effective concentration (EC) was calculated. 50 ). Meanwhile, the cytotoxic effects of compounds were assessed by assessing MCC (the minimum cytotoxic concentration that results in a microscopically detectable change in cell morphology). The effect of compounds on cell growth was determined by counting the number of cells in mock-infected cultures using a Coulter counter and expressed as the cytostatic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com