Benzisoselenazolone derivative, preparation method and application in anti-coronavirus drugs
A technology of benzisoselenazolone and benzisoselenazole, applied in the field of anti-coronavirus drug discovery, can solve problems such as lack of targeted Mpro active compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
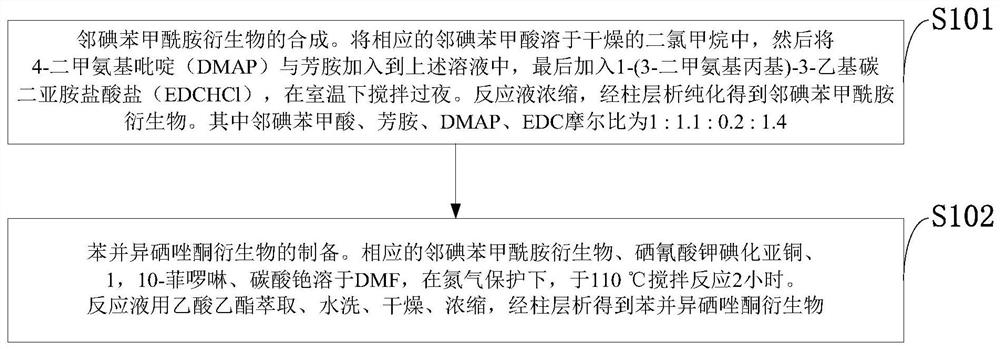
[0130] Such as figure 1 As shown, the preparation method of the benzisoselenazolon derivative provided by the invention comprises the following steps:
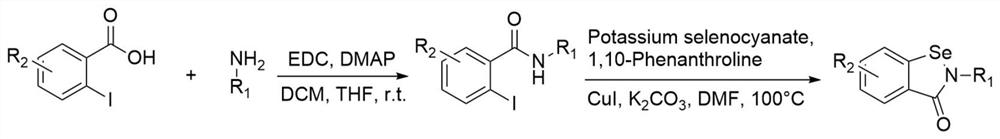
[0131] S101: Synthesis of o-iodobenzamide derivatives. Dissolve the corresponding o-iodobenzoic acid in dry dichloromethane, then add 4-dimethylaminopyridine (DMAP) and aromatic amine to the above solution, and finally add 1-(3-dimethylaminopropyl)- 3-Ethylcarbodiimide hydrochloride (EDC·HCl), stirred overnight at room temperature. The reaction solution was concentrated and purified by column chromatography to obtain o-iodobenzamide derivatives. The molar ratio of o-iodobenzoic acid, arylamine, DMAP, and EDC is 1:1.1:0.2:1.4.
[0132] S102: Preparation of benzisoselenazolone derivatives. The corresponding o-iodobenzamide derivatives, potassium selenocyanate cuprous iodide, 1,10-phenanthroline and cesium carbonate were dissolved in DMF, and stirred and reacted at 110°C for 2 hours under the protection of nitrogen. The rea...
Embodiment 1
[0136] Embodiment 1: Preparation of representative compound EB2-1 of benzisoselenazolone derivative
[0137]
[0138] Dissolve 2-iodo-benzoic acid (5 mmol) in dry dichloromethane, then add 4-dimethylaminopyridine (DMAP, 1 mmol) and 4-aminoaniline (5.5 mmol) to the above solution, and finally add 1 -(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl, 7 mmol), stirred overnight at room temperature. The reaction solution was concentrated and purified by column chromatography to obtain 2-iodo-N-(4-aminophenyl)benzamide.
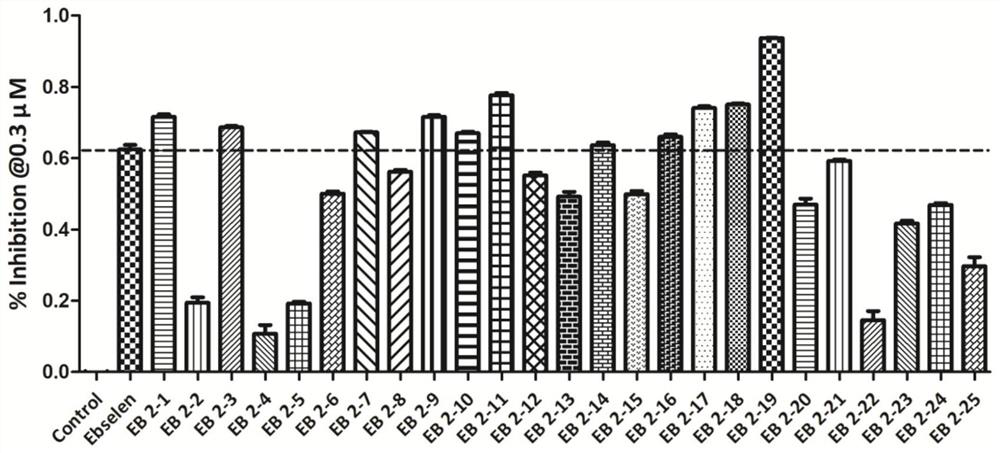
[0139] 2-iodo-N-(4-aminophenyl)benzamide (1.3mmol), potassium selenocyanate (1.56mmol), cuprous iodide (1.95mmol), 1,10-phenanthroline (1.95mmol) 1. Cesium carbonate (3.25 mmol) was dissolved in 3 ml of DMF, and stirred and reacted at 110° C. for 2 hours under the protection of nitrogen. The reaction solution was extracted with ethyl acetate, washed with water, dried, concentrated, and subjected to column chromatography to obtain the ebselen ...
Embodiment 2
[0140] Embodiment 2: Preparation of representative compound EB2-3 of benzisoselenazolone derivative
[0141]
[0142] Dissolve 2-iodobenzoic acid (5mmol) in dry dichloromethane, then add 4-dimethylaminopyridine (DMAP, 1mmol) and 3-aminoaniline (5.5mmol) to the above solution, and finally add 1- (3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl, 7 mmol), stirred overnight at room temperature. The reaction solution was concentrated and purified by column chromatography to obtain 2-iodo-N-(3-aminophenyl)benzamide.
[0143] 2-iodo-N-(3-aminophenyl)benzamide (1.3mmol), potassium selenocyanate (1.56mmol), cuprous iodide (1.95mmol), 1,10-phenanthroline (1.95mmol) 1. Cesium carbonate (3.25 mmol) was dissolved in 3 ml of DMF, and stirred and reacted at 110° C. for 2 hours under the protection of nitrogen. The reaction solution was extracted with ethyl acetate, washed with water, dried, concentrated, and subjected to column chromatography to obtain the ebselen seri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com