Application of compound for mediating cbl to degrade P-TFEB and exciting TFEB in preparation of antitumor drugs
A technology of P-TFEB and antineoplastic drugs, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
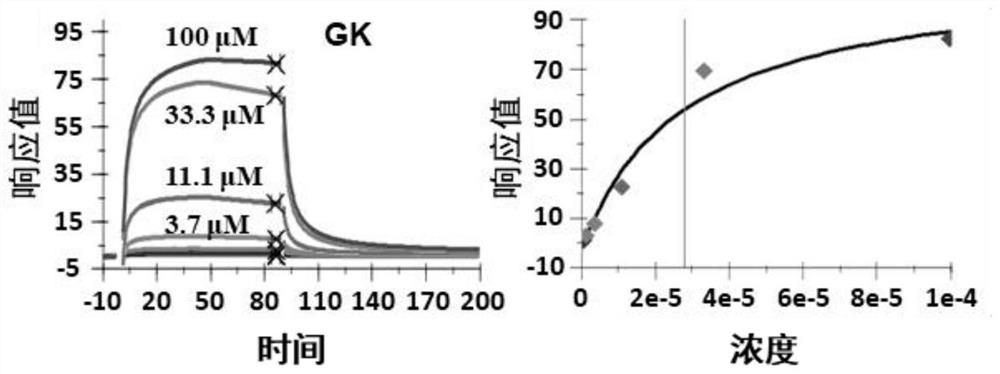
[0024] Example 1: Ginkgo biflavonoids bind to TFEB protein
[0025] The TFEB protein and its truncates were covalently immobilized on the CM5 chip through amine groups, and the immobilization level was 6500RU. Ginkgo biflavonoids solutions of different concentrations (100 μM, 33.3 μM, 11.1 μM, 3.7 μM, 1.2 μM, 0.41 μM, 0.14 μM) flowed over the chip surface to collect interaction signals in real time. The entire system was performed in 10 mM HEPES, pH 7.4, 150 mM NaCl, 0.05% P2O, 2% DMSO buffer solution. GE Life Sciences Biacore S200 system was used for detection and data analysis. The results showed that Ginkgo biflavonoids had a binding effect with TFEB protein ( figure 1 ).
Embodiment 2
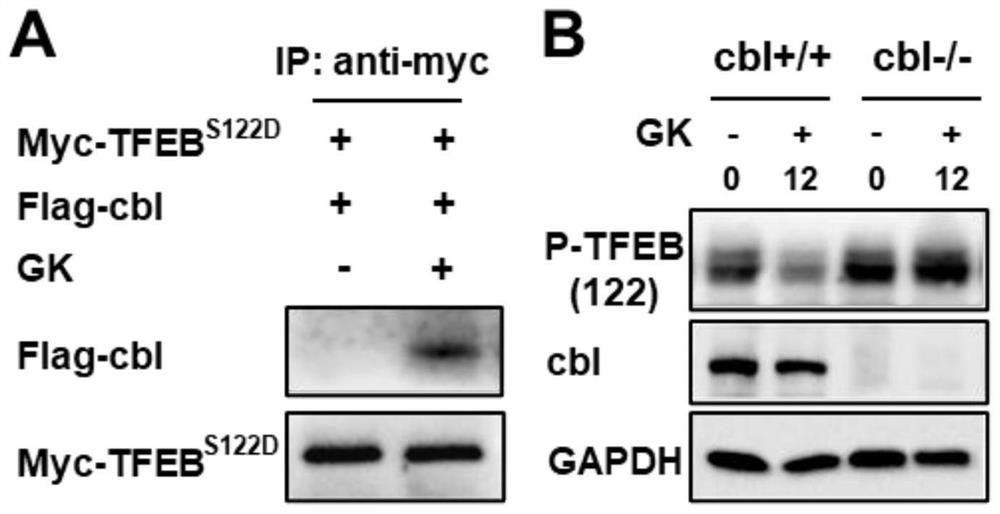
[0026] Example 2: Ginkgo biflavonoids induce P-TFEB to bind to cbl, and cbl is positively correlated with Ginkgo biflavonoids-induced P-TFEB degradation
[0027] A549 cells were cultured in 1640 medium containing 10% fetal bovine serum, penicillin 100U / ml, gentamicin 100ug / ml, and the incubator temperature was 37°C, containing 5% CO 2 . Using molecular cloning technology, the phosphorylation site S122 of TFEB was mutated to construct the phosphorylation site mutant plasmid pcDNA3.1-TFEB S122D , mimicking the persistent phosphorylation of TFEB.
[0028] Combining pcDNA3.1, pcDNA3.1-TFEB with pcDNA3.1-TFEB S122D They were transfected into A549 cells and given Ginkgo biflavonoids. Then cells were collected, lysed to extract protein, incubated with TFEB antibody and protein G-beads, washed protein G-beads with buffer 4 times, and dissolved the protein with urea. The peptides were then digested with trypsin and subjected to proteomic analysis by LC-MS / MS. The results show that...
Embodiment 3
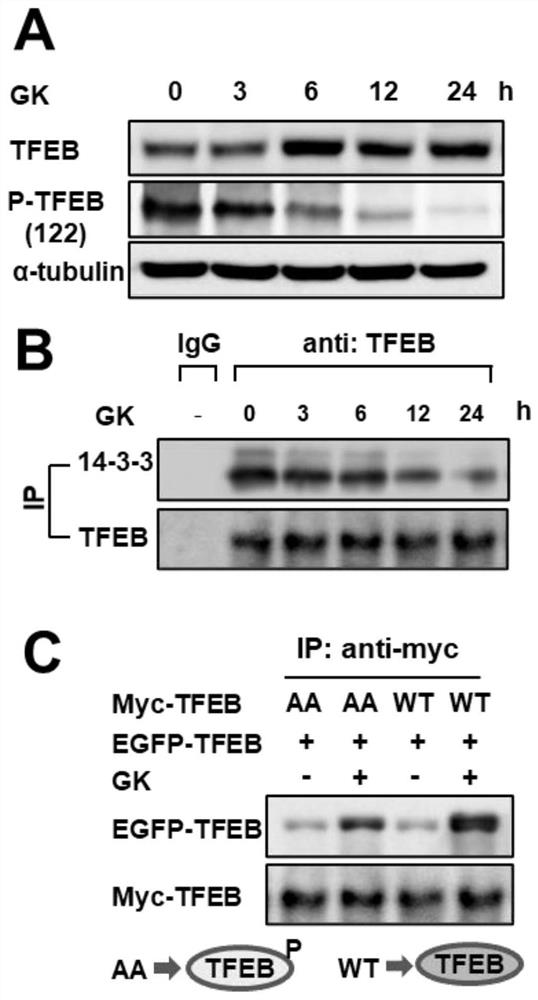
[0029] Example 3: Ginkgo biflavonoids promote TFEB phosphorylation, dissociate from binding protein 14-3-3, and promote TFEB / TFEB dimer formation.
[0030] A549 cells were collected at different time (3, 6, 12, 24h) after administration of Ginkgo biflavonoids, the cells were lysed, the protein was extracted, and the changes of P-TFEB were detected by western blot. The level of TFEB ( image 3 A).
[0031]The decrease in the phosphorylation level of TFEB will lead to its separation from the binding protein 14-3-3, and three forms of dimers will be formed between free TFEB and P-TFEB, namely P-TFEB dimer and TFEB dimer. polymer, P-TFEB\TFEB heterodimer. Among them, the TFEB dimer can enter the nucleus to play the function of transcription factor, and the appearance of P-TFEB in the dimer will affect the activation of TFEB activity. Immunoprecipitation was used to observe the binding of TFEB to 14-3-3 after Ginkgo biflavonoids were administered to A549 cells at different time ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com