Pegylated long-acting growth hormone as well as preparation method and medical application thereof
A polyethylene glycol, growth hormone technology, applied in growth hormone, chemical instruments and methods, hormone peptides, etc., to achieve the effect of improving pharmacokinetic properties, better stability, and good intermolecular consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Preparation and purification of rhGH samples modified with different branched polyethylene glycol propionaldehyde
[0068] 1. Sample preparation
[0069] (1) Preprocessing:
[0070] Take the original rhGh protein (secreted and expressed in the periplasm of Escherichia coli, the sequence is consistent with the natural sequence), treated with a 10kDa ultrafiltration membrane bag, the replacement buffer is sodium dihydrogen phosphate / disodium hydrogen phosphate buffer pH 6.0, and concentrated to the protein Concentration 10mg / mL.
[0071] (2) Feeding modification
[0072] PEG30K-rhGH modification preparation: add PEG to the pretreated rhGH protein solution according to the ratio of recombinant human growth hormone (hereinafter referred to as rhGH) and Y-PALD-PEG 30K molar ratio of 1:2, press PEG and reducing agent (cyano group) Sodium borohydride) in a molar ratio of 1:50, add a reducing agent to the mixed solution of PEG30K and protein, stir slowly until the ...
Embodiment 2
[0091] Example 2 Preparation and purification of Y-PEG-NHS modified rhGH samples
[0092] 1. Sample preparation
[0093] With reference to Chinese invention patent CN101385858A, the preparation of Y-PEG-NHS 40K random single-site modified rhGH samples was carried out. The specific plans are as follows:
[0094] The rhGh original protein was taken and treated with a 10kDa ultrafiltration membrane bag. The replacement buffer was 50mM sodium dihydrogen phosphate / disodium hydrogen phosphate buffer pH 6.5-6.8, and at the same time, it was concentrated to a protein concentration of 10 mg / mL.
[0095]The PEG modifier is polyethylene glycol-N-hydroxysuccinimidyl ester (trade name Y-PEG-NHS 40K, purchased from Beijing Jiankai Technology Co., Ltd.), and PEG is carried out according to the protein:PEG mass ratio of 1:6 Feeding, stirring was continued until the Y-PEG-NHS was completely dissolved, and the reaction was carried out at 4° C. for 16 hours to obtain a reaction mixture.
[00...
Embodiment 3
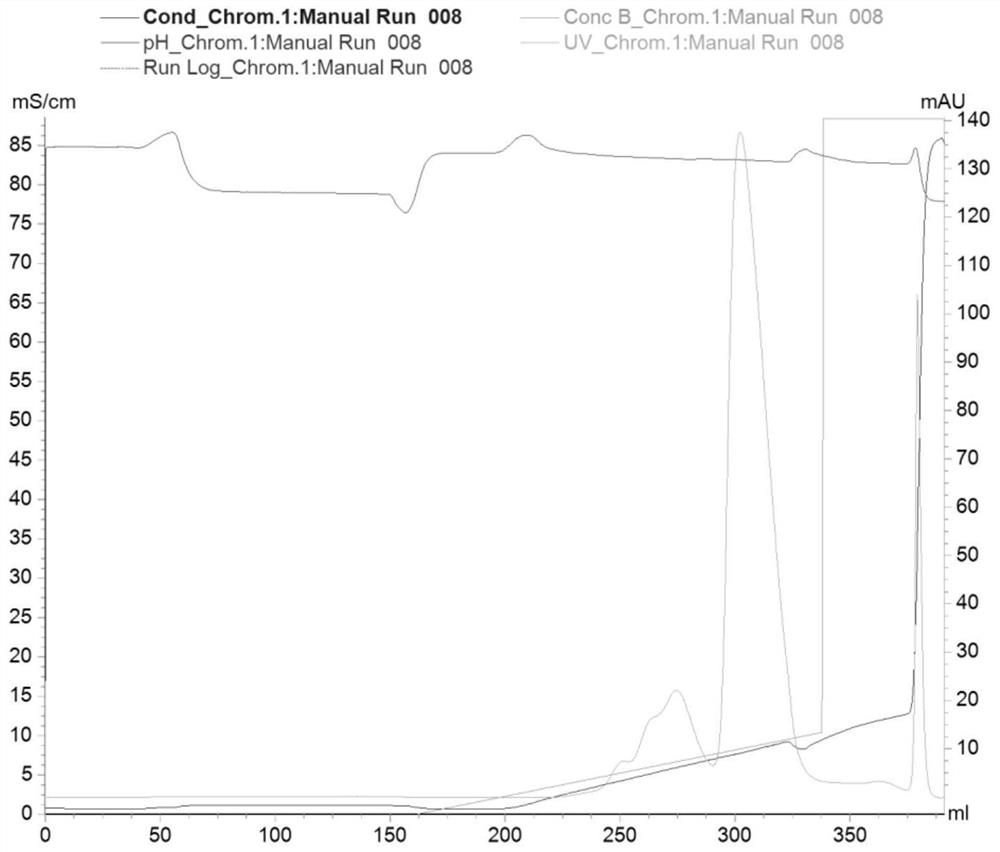
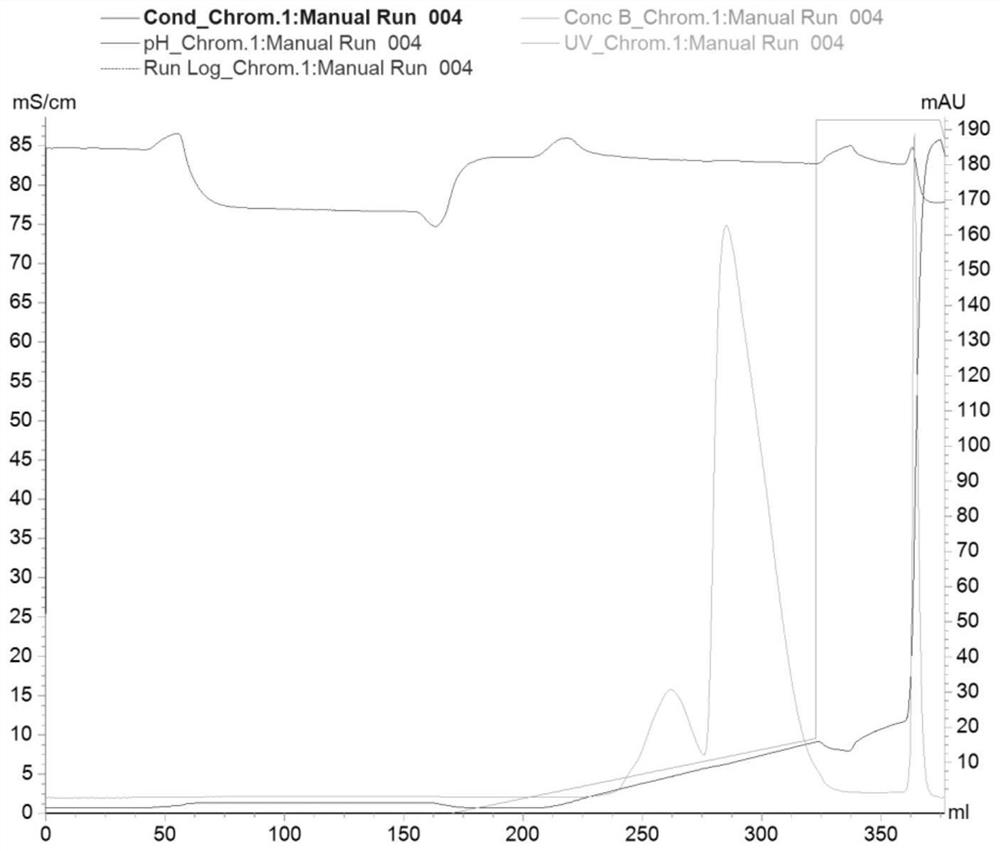
[0111] Example 3 Determination of the binding number of rhGH PEG modified with different PEGs
[0112] In the present invention, the following method can be used to compare the number of PEG-binding molecules of GH in different PEG-modified rhGH.
[0113] Take PEG60K-rhGH as an example:
[0114] 1. Detection method
[0115] Use liquid chromatography differential-UV combined detection method.
[0116] The detection column is BEH SEC 3.5um, 7.8*300mm. The mobile phase was 20mM pb7.0+5% isopropanol, the column temperature was set to 35°C, and 10ul of sample was loaded. The collection time was set to 30min; the temperature of the flow cell was set to 35°C; the flow rate of the liquid phase was set to 0.5ml / min. The detector used RI+UV (collection wavelength 280 nm).
[0117] The sample processing method is as follows: PEG60K-rhGH and GH proprotein are respectively diluted to 1.0mg / ml with pH7.0 phosphate buffer; 5mg of PEG60K is accurately weighed, accurately dissolved with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com