Method for synthesizing Piragliatin and analogues thereof
A technology of analogs and complexes, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problems of lengthy steps, environmental pollution, increase production cost, etc., and achieve the effect of cheap synthesis and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
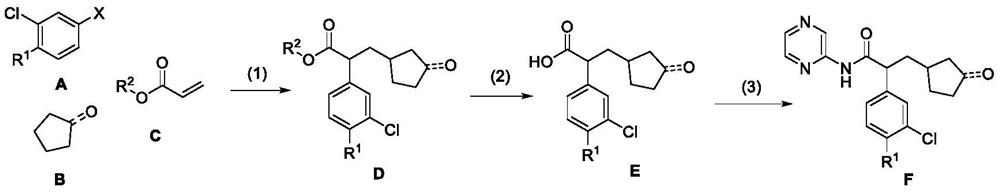
[0031] The synthesis path is as follows:
[0032]
[0033] The preparation method is as follows:
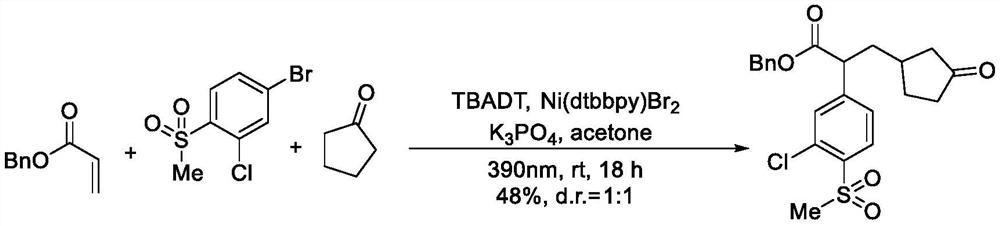
[0034] Under an inert atmosphere, tetrabutylammonium decatungstate (13.3 mg, 0.004 mmol), (4,4'-di-tert-butyl-2,2'-bipyridine) nickel dibromide (9.8 mg) were added to the reaction tube. , 0.02mmol), potassium phosphate (84.8mg, 0.4mmol), benzyl acrylate (97.2mg, 0.6mmol), cyclopentanone (168.2mg, 2mmol), 4-bromo-2-chloro-1-(methylsulfonic acid) acyl)benzene (53.9 mg, 0.2 mmol) and acetone (0.5 mL). Under the irradiation of a violet lamp (10w, 390nm), the reaction was carried out at room temperature for 18 hours, concentrated, and subjected to flash column chromatography to obtain the product 2-(3-chloro-4-(methylsulfonyl)phenyl)-3-(3-oxocycle) Benzyl pentyl)propionate 41.8 mg (yield 48%, d.r.=1:1, colorless transparent liquid.
[0035] 1 H NMR (600MHz, CDCl 3 )δ8.11-8.08(m,1H),7.54-7.51(m,1H),7.42-7.38(m,1H),7.37-7.31(m,4H),7.28-7.26(m,1H),5.19- 5.15(m, 1H), 5.11-5.07(m,...
Embodiment 2
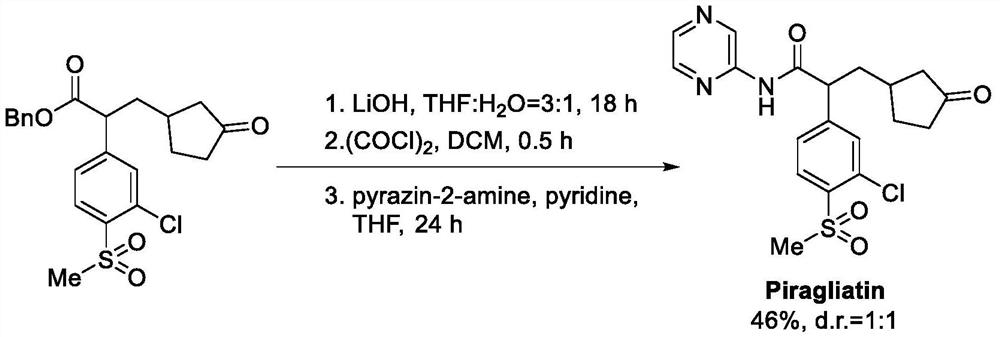
[0043] The synthesis path is as follows:
[0044]
[0045] The preparation method is as follows:
[0046] Under an inert atmosphere, tetrabutylammonium decatungstate (13.3 mg, 0.004 mmol), (4,4'-di-tert-butyl-2,2'-bipyridine) nickel dibromide (9.8 mg) were added to the reaction tube. , 0.02 mmol), potassium phosphate (84.8 mg, 0.4 mmol), methyl acrylate (51.7 mg, 0.6 mmol), cyclopentane (140.2 mg, 2 mmol), 4-bromo-2-chloro-1-(methylsulfonic acid) acyl)benzene (53.9 mg, 0.2 mmol) and acetone (0.5 mL). Under the irradiation of a violet lamp (10w, 390nm), the reaction was carried out at room temperature for 18 hours, concentrated, and subjected to flash column chromatography to obtain the product, methyl 2-(3-chloro-4-(methylsulfonyl)phenyl)-3-cyclopentylpropanoate Ester 31.0 mg (yield 45%, colorless transparent liquid).
[0047] 1 H NMR (600MHz, CDCl 3 )δ8.08(d,J=8.2Hz,1H),7.53(d,J=1.7Hz,1H),7.44-7.37(m,1H),3.68(s,3H),3.66(t,J=7.8 Hz, 1H), 3.26(s, 3H), 2.12-2.05(m, 1H),...
Embodiment 3
[0055] The synthetic route is as follows:
[0056]
[0057] The preparation method is as follows:
[0058] Under an inert atmosphere, tetrabutylammonium decatungstate (13.3 mg, 0.004 mmol), (4,4'-di-tert-butyl-2,2'-bipyridine) nickel dibromide (9.8 mg) were added to the reaction tube. , 0.02mmol), potassium phosphate (84.8mg, 0.4mmol), methyl acrylate (51.7mg, 0.6mmol), cyclopentane (140.2mg, 2mmol), 4-bromo-2-chloro-1-fluorobenzene (41.8 mg, 0.2 mmol) and acetone (0.5 mL). Under the irradiation of a violet lamp (10w, 390nm), the reaction was performed at room temperature for 18 hours, concentrated, and subjected to flash column chromatography to obtain 30.6 mg of the product, methyl 2-(3-chloro-4-fluorophenyl)-3-cyclopentylpropionate (yield 30.6 mg). The rate is 54%, colorless and transparent liquid).
[0059] 1 H NMR(600MHz, CDCl3)δ7.37(dd,J=7.0,2.3Hz,1H),7.20-7.16(m,1H),7.08(t,J=8.7Hz,1H),3.67(s,3H) ,3.56(t,J=7.8Hz,1H),2.07-2.01(m,1H),1.80-1.70(m,3H),1.65-1.60(m,2H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com