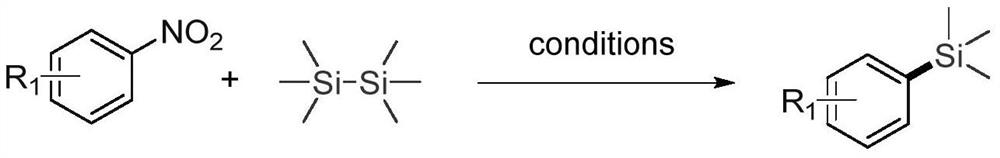
Synthesis method of novel aryl silane compound
A technology of aryl silane and synthesis method, which is applied in the field of synthesis of new aryl silane compounds, and can solve the problems of increased cost, low atom economy, environmental pollution of organic halide waste, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
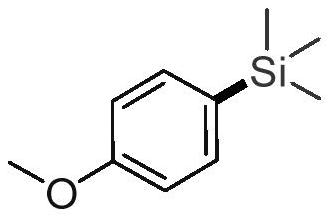
[0021] Add 0.3 mmol p-nitroanisole, 0.2 mmol hexamethyldisilane, 0.01 mmol palladium acetylacetonate, 0.02 mmol BrettPhos, 0.6 mmol cesium carbonate, 1.5 mL trifluorotoluene to a Schlenk tube. at 150°C, N 2 After stirring and reacting under the same conditions for 24 hours, stop heating and stirring, and cool to room temperature. Suction filtration, removal of the solvent under reduced pressure, separation and purification by column chromatography to obtain the target product. The volume ratio of the column chromatography eluent used was 50:1 petroleum ether:ethyl acetate mixed solvent.
[0022] The structural characterization data of the resulting product are as follows:
[0023] 1 H NMR (500MHz, CDCl 3 )δ7.49(d, J=8.6Hz, 2H), 6.95(d, J=8.6Hz, 2H), 3.84(s, 3H), 0.29(s, 9H);
[0024] 13 C NMR (126MHz, CDCl 3 )δ160.2, 134.7, 131.2, 113.5, 54.9, -1.0.
[0025] According to the above data, the structure of the resulting product is deduced as shown in the following formula...
Embodiment 2
[0028] Add 0.3 mmol 2-nitronaphthalene, 0.2 mmol hexamethyldisilane, 0.01 mmol palladium acetylacetonate, 0.02 mmol BrettPhos, 0.6 mmol cesium carbonate, 1.5 mL trifluorotoluene to a Schlenk tube. at 150°C, N 2 After stirring and reacting under the same conditions for 24 hours, stop heating and stirring, and cool to room temperature. Suction filtration, removal of the solvent under reduced pressure, separation and purification by column chromatography to obtain the target product. The eluent used in column chromatography was petroleum ether.
[0029] The structural characterization data of the resulting product are as follows:
[0030] 1 H NMR (500MHz, CDCl 3 )δ8.03(s,1H),7.85(ddd,J=11.7,5.9,3.2Hz,3H),7.62(dd,J=8.1,1.1Hz,1H),7.51–7.47(m,2H),0.37 (s,9H).
[0031] 13 C NMR (126MHz, CDCl 3 )δ137.9, 133.7, 133.6, 132.9, 129.8, 128.0, 127.7, 126.9, 126.2, 125.8, -1.1
[0032] According to the above data, the structure of the resulting product is deduced as shown in the fol...
Embodiment 3
[0035] Add 0.3 mmol N,N-dimethyl-4-nitroaniline, 0.2 mmol hexamethyldisilane, 0.01 mmol palladium acetylacetonate, 0.02 mmol BrettPhos, 0.6 mmol cesium carbonate, 1.5 mL benzotrifluoride to a Schlenk tube. at 150°C, N 2 After stirring and reacting under the same conditions for 24 hours, stop heating and stirring, and cool to room temperature. Suction filtration, removal of the solvent under reduced pressure, separation and purification by column chromatography to obtain the target product. The volume ratio of the column chromatography eluent used was 40:1 petroleum ether:ethyl acetate mixed solvent.
[0036] The structural characterization data of the resulting product are as follows:
[0037] 1 H NMR (500MHz, CDCl 3 )δ7.46(d, J=8.6Hz, 2H), 6.80(d, J=8.6Hz, 2H), 3.01(s, 6H), 0.29(s, 9H).
[0038] 13 C NMR (126MHz, CDCl 3 )δ151.1, 134.5, 125.8, 112.1, 40.4, -0.7.
[0039] According to the above data, the structure of the resulting product is deduced as shown in the foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com