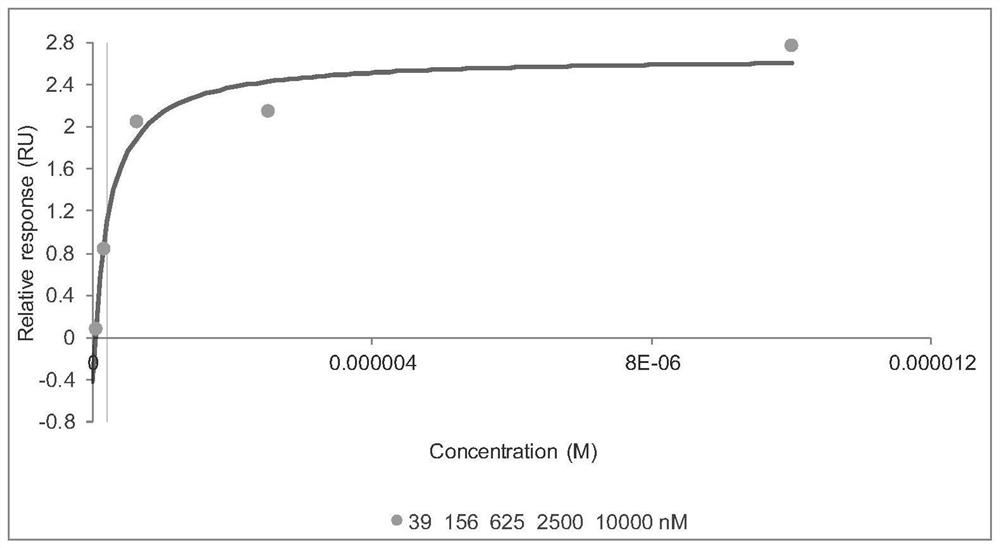
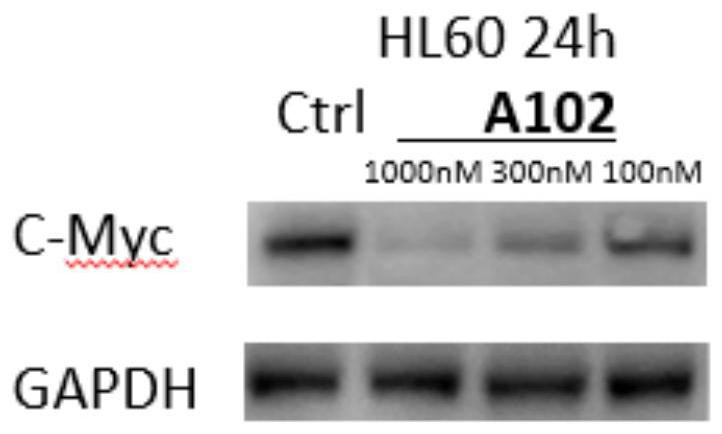
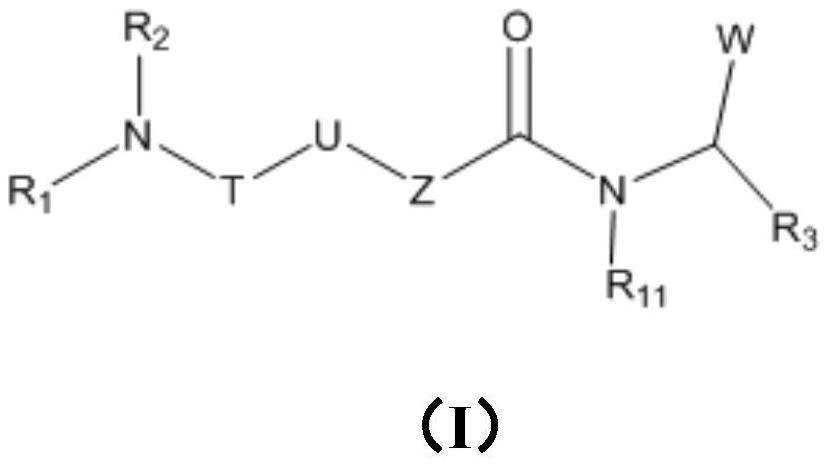
C-Myc protein inhibitor as well as preparation method and application thereof
A solvent compound, C1-C3 technology, applied in the field of medicine, can solve problems such as c-Myc inhibitors that have not been reported, and achieve excellent c-Myc protein inhibitory effect, simple synthesis method, and exact inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Synthesis of Compound A1
[0093] Compound A1:
[0094]
[0095]
[0096] Compounds A1-1 (156mg, 0.5mmol) and A1-2 (227mg, 0.6mmol) were dissolved in DCM (3mL), HATU (285mg, 0.75mmol) and TEA (151mg, 1.5mmol) were added and reacted at room temperature for two hours . Water was added, extracted with DCM (15mL*3), the organic phases were combined, the solvent was pulled dry, and the crude product was purified by medium pressure preparation to obtain compound A1 (28mg, yield 10%) as a white solid.
[0097] 1 H NMR (400MHz, DMSO) δ9.28 (s, 1H), 7.90 (d, J = 7.5Hz, 2H), 7.68 (d, J = 7.3Hz, 2H), 7.49–7.37 (m, 2H), 7.34 (t,J=7.2Hz,3H),4.30(dd,J=12.2,6.6Hz,2H),4.22(t,J=6.8Hz,1H),4.00(d,J=6.8Hz,1H),3.21 (dd,J=13.2,6.8Hz,2H),2.46–2.39(m,2H),2.17(dd,J=12.2,10.1Hz,1H),2.01(dd,J=14.6,6.9Hz,2H), 1.85–1.75(m,2H),1.74–1.65(m,1H),1.61(d,J=14.1Hz,1H),1.36(dd,J=12.7,10.3Hz,1H),1.25(dd,J= 19.3,15.2Hz,8H),0.91–0.75(m,9H).
[0098] Compounds A2-A19, 28-41, 44-55, 57-58, 66-69, 9...
Embodiment 2
[0271] Synthesis of compound A72
[0272] Compound A72:
[0273]
[0274] Synthesis of compound A72-2
[0275] Compound A72-1 (150mg, 0.617mmol) was dissolved in a mixed solvent (2mL) of toluene / methanol=1 / 3, and TMSCHN2 (2mL, 0.6M, 1.23mmol) was added under stirring at room temperature, and the reaction solution was light yellow and clear After stirring at room temperature for 5 min, TLC showed that the reaction was complete. The reaction solution was spin-dried, and dichloromethane (3 mL) and trifluoroacetic acid (1 mL) were added. After stirring at room temperature for 1 h, the reaction was complete, and the solvent was spin-dried to obtain the crude compound A72-2 ( 250mg, crude product).
[0276] Synthesis of compound A72-4
[0277] Compound A72-2 (250 mg, crude product), compound A72-3 (120 mg, 0.53 mmol) and HATU (243 mg, 0.63 mmol) were dissolved in dichloromethane (8 mL), and triethylamine (235 uL, 1.7 mmol), after stirring at room temperature for 2 hours, TLC ...
Embodiment 3
[0343] Embodiment 3: the synthesis of compound A91
[0344] Compound A91
[0345]
[0346] Compounds A91-1 (176mg, 0.5mmol) and A91-2 (202mg, 0.6mmol) were dissolved in DCM (3mL), HATU (285mg, 0.75mmol) and TEA (151mg, 1.5mmol) were added and reacted at room temperature for two hours . Add water, extract with DCM (15mL*3), combine the organic phases, pull dry the solvent, prepare and purify the crude product with medium pressure to obtain white solid compound A91 (17mg, yield 6%)
[0347] 1H NMR (400MHz, DMSO) δ9.48 (d, J = 10.6Hz, 1H), 7.89 (d, J = 7.5Hz, 2H), 7.72–7.63 (m, 2H), 7.44–7.25 (m, 5H) ,4.25(dt,J=17.6,5.2Hz,3H),3.94(t,J=6.9Hz,1H),3.23(dd,J=15.5,8.5Hz,1H),2.39(d,J=7.3Hz, 1H),2.20–2.09(m,1H),1.98(d,J=21.6Hz,1H),1.77(dd,J=11.0,5.4Hz,2H),1.58(d,J=13.5Hz,1H), 1.39–1.32(m,1H),1.21(t,J=7.8Hz,8H),0.98(dd,J=7.0,4.1Hz,3H),0.86(t,J=10.8Hz,1H),0.78(s ,3H),0.42–0.27(m,2H),0.22(d,J=3.5Hz,2H).
[0348] The synthetic method of compound 92 is the same as that of A91.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com