Production process of guanidine sulfate
A production process and technology of guanidine sulfate, applied in the field of preparation of guanidine-based compounds, can solve the problems of no mature guanidine sulfate production process, unstable sulfate ion, low product purity, etc., and achieve simple and controllable reaction principle, less impurities and purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
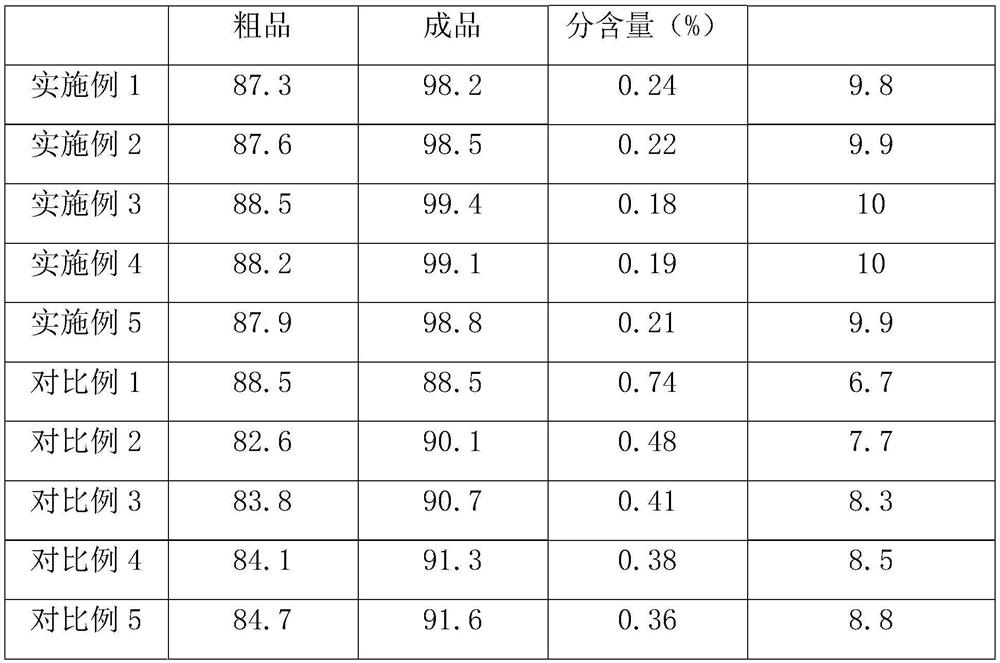
Examples
Embodiment 1
[0047] Embodiment 1: Guanidine sulfate is prepared by the following method:
[0048] S1. Mix guanidine hydrochloride with a molar ratio of 2:1 and sulfuric acid with a mass fraction of 80%, place guanidine hydrochloride in sulfuric acid at a constant speed within 60s, and stir continuously at a stirring speed of 300r / min during this period, and then place it at a temperature of 0°C React 48h under condition, generate reaction product and hydrogen chloride gas;
[0049] S2. Suction filter the reaction product prepared in S1 while it is hot, take the filtrate and cool it down to 10°C at a cooling rate of 5°C / 10min, then let it stand for 2 hours, and obtain crude guanidine sulfate after filtration; pass hydrogen chloride gas into the hydrogen oxidizer In sodium aqueous solution, sodium chloride solution and water are generated;
[0050] S3, put the crude product guanidine sulfate prepared in S2 into water and stir and dissolve for 15min under the condition of 400r / min to obtain ...
Embodiment 2
[0052] Embodiment 2: Guanidine sulfate is prepared by the following method:
[0053] S1, mixing guanidine hydrochloride with a molar ratio of 2:1.2 and sulfuric acid with a mass fraction of 88%, placed guanidine hydrochloride in sulfuric acid at a uniform speed within 60s, during which it was constantly stirred at a stirring speed of 300r / min, and then heated at 50°C React 24h under condition, generate reaction product and hydrogen chloride gas;
[0054] S2. Suction filter the reaction product prepared in S1 while it is hot, take the filtrate and cool it down to 12°C at a cooling rate of 5°C / 10min, then let it stand for 2.5h, and obtain crude guanidine sulfate after filtration; pass hydrogen chloride gas into hydrogen In the aqueous solution of sodium oxide, sodium chloride solution and water are generated;
[0055] S3, put the crude product guanidine sulfate prepared in S2 into water and stir and dissolve under the condition of 450r / min for 18min to obtain a stirred liquid; ...
Embodiment 3
[0057] Embodiment 3: Guanidine sulfate is prepared by the following method:
[0058] S1, the guanidine hydrochloride that the molar ratio is 2:1.5 and the concentrated sulfuric acid that mass fraction is 98% are mixed, and guanidine hydrochloride is placed in the concentrated sulfuric acid at a constant speed within 60s, constantly stirs with the stirring speed of 300r / min during this period, then in 72 Under the condition of ℃, react for 8 hours to generate reaction product and hydrogen chloride gas;
[0059] S2. Suction filter the reaction product prepared in S1 while it is hot. Take the filtrate and cool it down to 50°C at a cooling rate of 5°C / 10min. Stir continuously at a speed of 450r / min during this period. Stop stirring when the temperature is lower than 50°C. Continue Cool down to 15°C at a cooling rate of 5°C / 10min, then stand still for 3 hours, and obtain crude guanidine sulfate after filtration; pass hydrogen chloride gas into aqueous sodium hydroxide solution to g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com