Composite probiotic composition for treating hyperuricemia and gout as well as preparation method and application thereof
A technology of compound probiotics and composition, applied in the field of probiotics, to achieve the effect of good gout symptoms, relief of gout symptoms, and lower blood uric acid level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A compound probiotic bacteria powder, including Lactobacillus casei Zhang (Lactobacillus casei Zhang), Bifidobacterium animalis subsp.lactis V9, Lactobacillus plantarum P-8, Lactobacillus rhamnosus Probio-M9 (Lactobacillus rhamnosus Probio-M9) and Bifidobacterium lactis Probio-M8 (Bifidobacterium lactis Probio-M8).
[0058] The microorganism preservation number of the Lactobacillus casei Zhang (Lactobacillus casei Zhang) is CGMCCNo.5469;
[0059] The microorganism preservation number of Bifidobacterium animalis subsp. lactis V 9 is CGMCC No.5470;
[0060] The microorganism preservation number of Lactobacillus plantarum P-8 is CGMCCNo.6312;
[0061] The microbial deposit number of Lactobacillus rhamnosus Probio-M9 is CGMCC No.18639;
[0062] The microorganism preservation number of Bifidobacterium lactis Probio-M8 (Bifidobacterium lactis Probio-M8) is CGMCC No.18610.
[0063] The colony-forming unit ratio of Lactobacillus casei Zhang, Bifidobacterium animalis subsp. V...
experiment example 1
[0084] Experimental Example 1 Clinical research on the adjuvant treatment of HUA with compound probiotic powder
[0085] 1 Experimental method
[0086] 1.1 Bacterial strains contained in the composite probiotic powder prepared in Example 1
[0087] L.casei Zhang, L.plantarum P-8, B.lactis V9, B.lactis Probio-M8 and L.rhamnosus Probio-M9 were provided by Beijing Ketuo Hengtong Biotechnology Co., Ltd.
[0088] 1.2 Test grouping
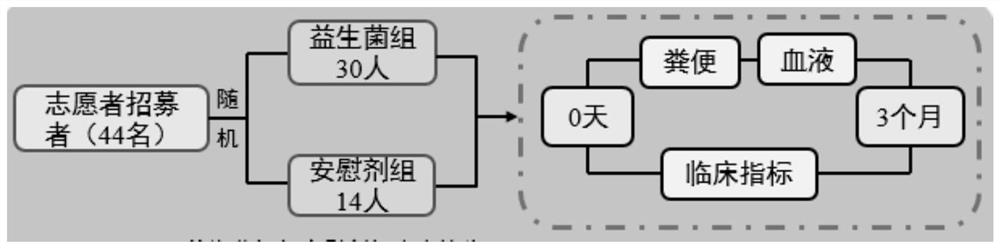
[0089] 44 patients with hyperuricemia were randomly divided into probiotic group (30 people) and placebo group (14 people). The probiotic group was given 30 billion CFU of compound probiotic powder every day.
[0090] Blood samples were collected before treatment and at 12 weeks of treatment to analyze changes in blood uric acid levels and related metabolic syndrome indicators (total cholesterol, triglycerides, low-density lipoprotein) levels. For specific experimental procedures, see figure 1 .
[0091] 1.3 Patient inclusion and exclusion criteria...
experiment example 2
[0105] Experimental Example 2 Clinical effect of compound probiotic powder in adjuvant treatment of gout
[0106] 1. Volunteer inclusion criteria
[0107] A. Age 18-70 years old, gender is not limited;
[0108] B. Previous history of gout attacks;
[0109] C. Meet the 2015 Eular / ACR gout classification diagnostic criteria;
[0110] D. Serum uric acid is 475-750 μmol / L.
[0111] 2. Exclusion criteria for volunteers
[0112] A. Patients with gout or secondary hyperuricemia in the past two weeks;
[0113] B. Taking drugs that affect uric acid metabolism or excretion and cannot / cannot be stopped;
[0114] C. Abnormal liver and kidney function, white blood cell count, platelet count, and hemoglobin level;
[0115] D. Patients with poorly controlled blood pressure, type 1 diabetes or poorly controlled type 2 diabetes;
[0116] E. Patients with intestinal diseases such as active peptic ulcer and malignant tumor patients;
[0117] F. Patients who need to continue taking predni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com