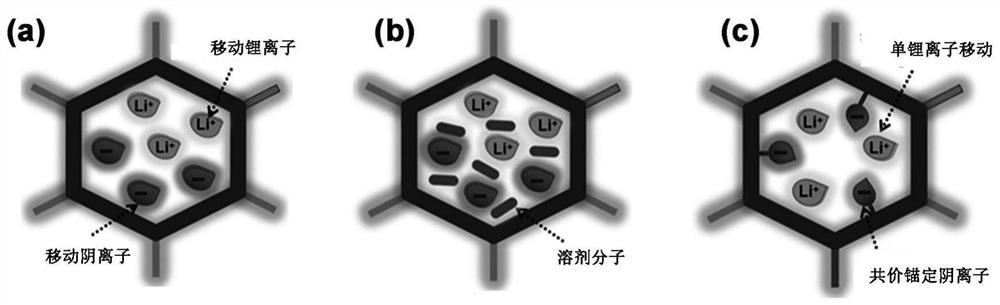
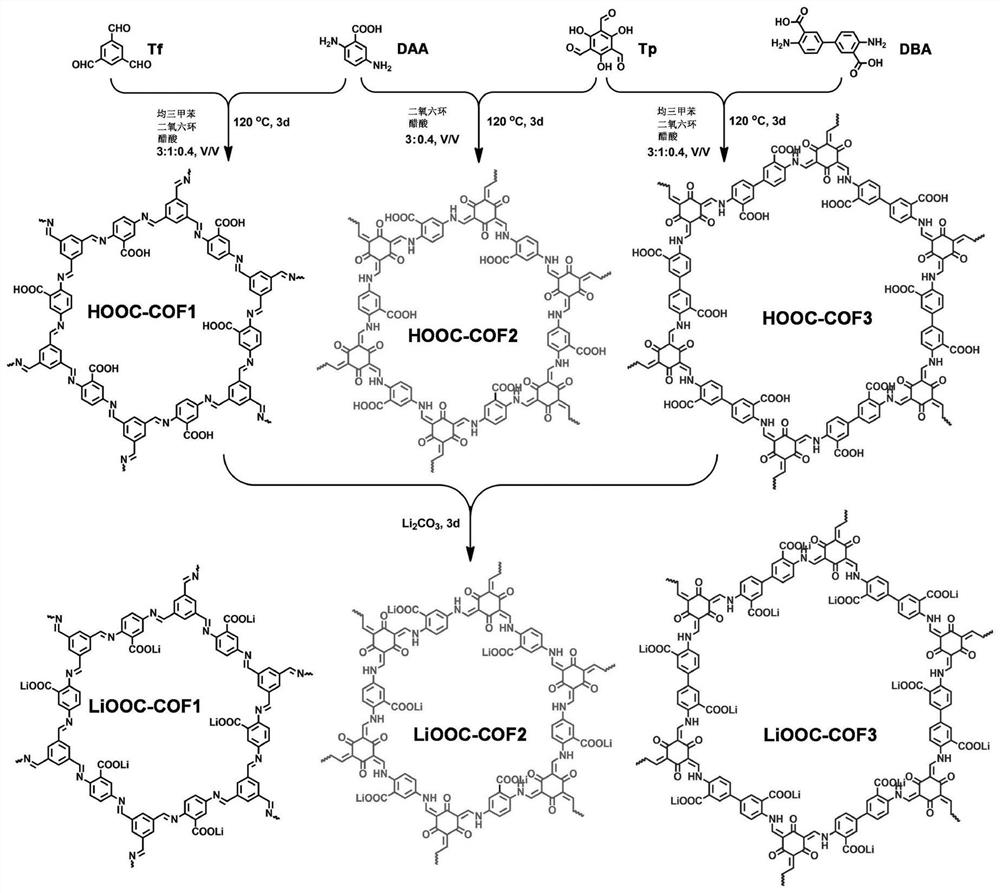
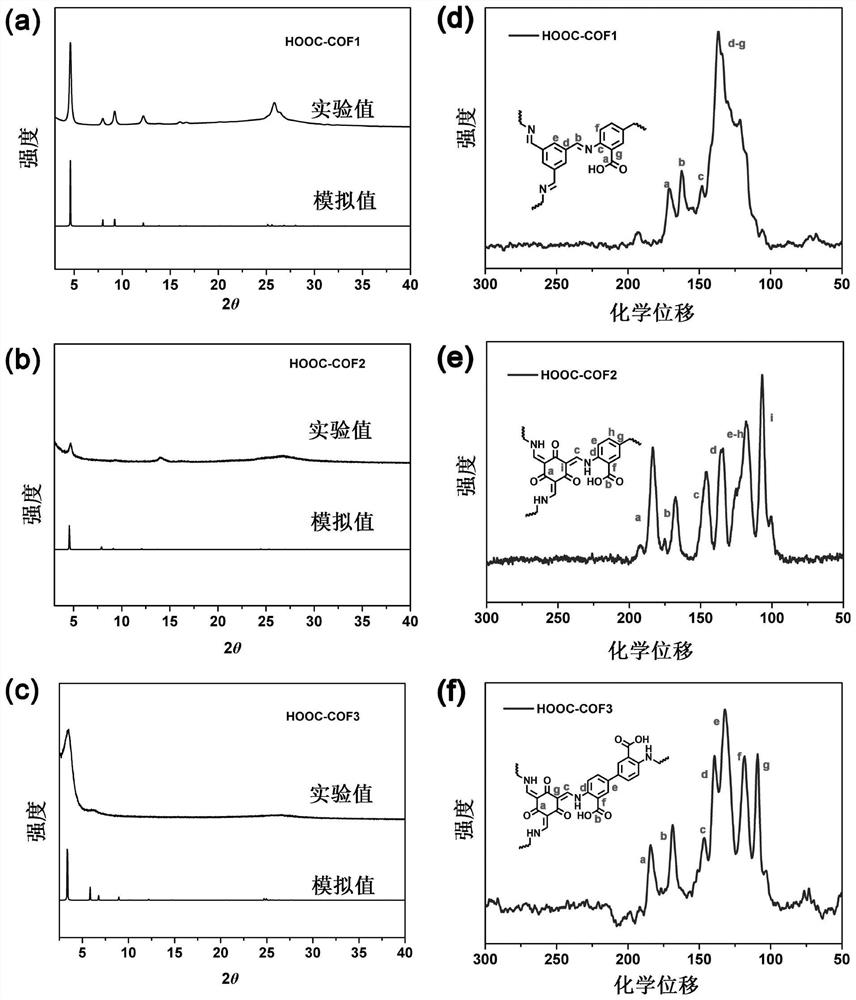
COFs (Covalent Organic Frameworks) solid electrolyte containing lithium carboxylate group as well as preparation method and application of COFs solid electrolyte
A solid electrolyte, lithium carboxylate technology, applied in circuits, electrical components, secondary batteries, etc., can solve problems such as hindering the movement and migration of lithium ions, adverse effects of lithium metal batteries, limiting lithium ion content, etc., to promote lithium ions. Effects of migration, elimination of interface side reactions, and shortening of transition distances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The invention provides a method for preparing a COFs solid-state electrolyte containing a lithium carboxylate group, comprising the following steps:
[0033] Mixing aldehyde-containing monomers, amino-containing organic carboxylic acid monomers, acid catalysts and organic solvents, and performing amine-aldehyde condensation reactions to obtain carboxyl-containing products;
[0034] The carboxyl group-containing product is mixed with a lithium salt solution for ion exchange to obtain a COFs solid state electrolyte containing lithium carboxylate groups.
[0035] In the present invention, unless otherwise specified, the required preparation materials are commercially available products well known to those skilled in the art.
[0036] The invention mixes aldehyde group-containing monomers, amino group-containing organic carboxylic acid monomers, acid catalysts and organic solvents to carry out amine-aldehyde condensation reaction to obtain carboxyl group-containing products...
Embodiment 1
[0060] Tritylaldehyde (Tf) (48.0 mg, 0.3 mmol), 2,5-diaminobenzoic acid (DAA) (68.5 mg, 0.45 mmol) and 4.0 mL of mesitylene / 1,4-dioxane ( 3:1, v / v) mixed solution into the ampoule, ultrasonic 10.0min, the CH 3 COOH (0.4mL, 6.0M) was added to the mixture obtained by ultrasound, the ampoule was frozen in liquid nitrogen, vacuum-sealed, sealed and placed in an oven at 120°C for 3 days. After cooling to room temperature, the resulting solid was collected by filtration and used separately After washing with tetrahydrofuran (THF) and acetone for 4 times, the obtained solid was soaked in anhydrous THF for 1 d, and the solvent was changed 4 times a day, and the obtained solid was vacuum-dried at 100 °C for 12 h to obtain a yellow powdery COF, which was designated as HOOC-COF1 ;
[0061] Incorporation of HOOC-COF1 into Li 2 CO 3 (20mL, 1.0M) aqueous solution, make HOOC-COF1 and Li 2 CO 3 The molar ratio is 1:100, stirred in a round-bottomed flask for 2 d, and the resulting solid w...
Embodiment 2
[0063] 1,3,5-Triformylphloroglucinol (Tp) (63.0 mg, 0.3 mmol), 2,5-diaminobenzoic acid (DAA) (68.5 mg, 0.45 mmol) and 3.0 mL of 1,4-dioxo The mixed solution of hexacyclic was added to the ampoule, ultrasonicated for 10.0min, and the CH 3 COOH (0.4mL, 6.0M) was added to the mixture obtained by ultrasound, the ampoule was frozen in liquid nitrogen, vacuum-sealed, sealed and placed in an oven at 120°C for 3 days. After cooling to room temperature, the resulting solid was collected by filtration and used separately After washing with tetrahydrofuran (THF) and acetone for 4 times, the obtained solid was soaked in anhydrous acetone for 1 d, and the solvent was changed 4 times a day, and the obtained solid was vacuum-dried at 100 °C for 12 h to obtain a red powdery COF, which was designated as HOOC-COF2 ;
[0064] Incorporation of HOOC-COF2 into Li 2 CO 3 (20.0mL, 1.0M) in aqueous solution, make HOOC-COF2 and Li 2 CO 3 The molar ratio is 1:100, stirred in a round-bottomed flask ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com