Synthesis process and application of hydroxyl-terminated hyperbranched polyphosphazene flame retardant
A technology of hydroxyl-terminated hyperbranching and polyphosphazene, which is applied in the direction of polyurea/polyurethane coatings, coatings, fireproof coatings, etc. To achieve the effects of enhancing thermal stability and heat resistance, inhibiting the reaction process, and enhancing flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
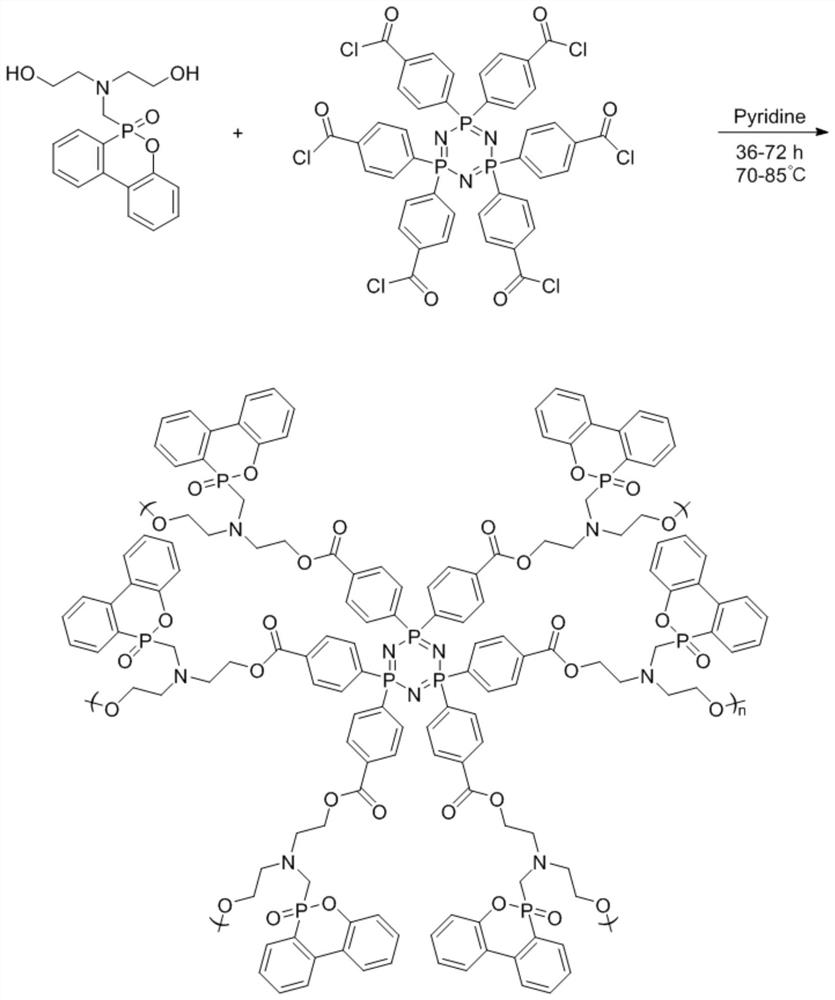
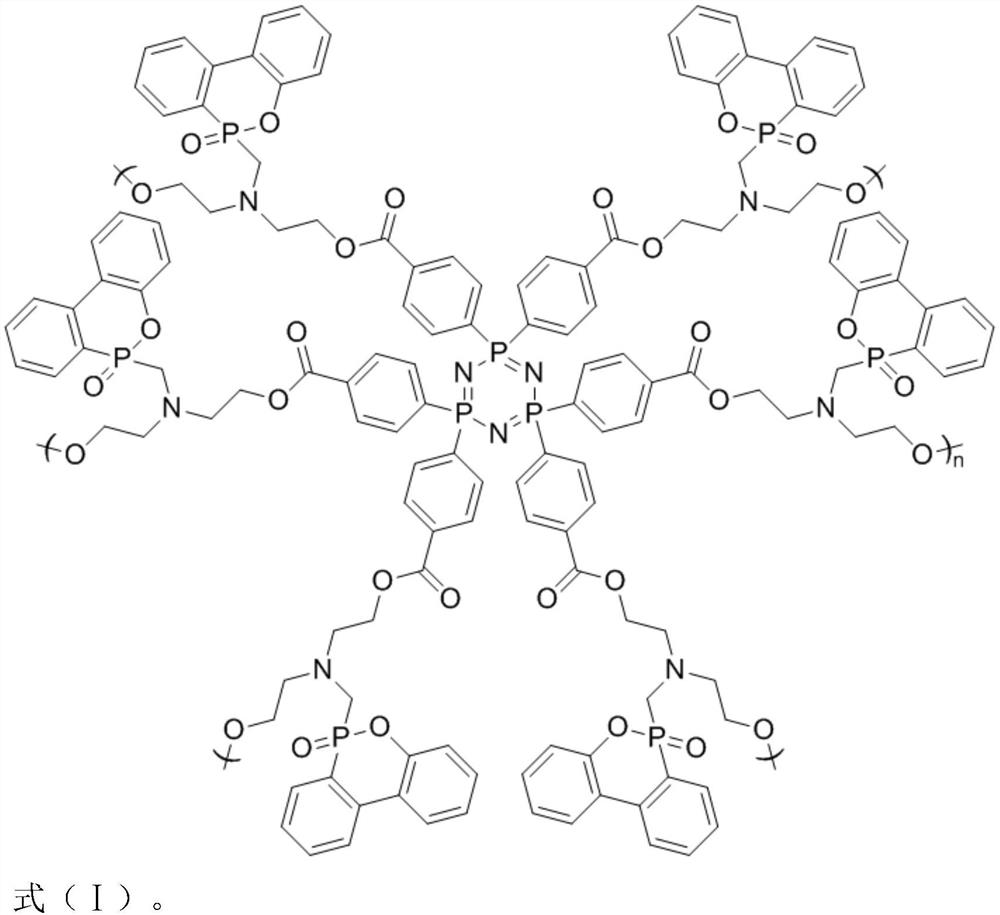
[0029] (1) Add 0.2 g of carboxycyclotriphosphazene to 5 mL of SOCl 2 , pass into N 2 Exhaust the air, heat to 60°C, react for 5 hours, and distill under reduced pressure after the reaction, wash the product with dichloromethane and acetone to obtain cyclotriphosphazene acid chloride.
[0030] (2) Add 0.5% of acid chloride cyclotriphosphazene into xylene solvent, stir to dissolve and then pass into N 2 Exhaust air, add 0.83g dihydroxy DOPO derivative (C 17 h 20 NO 4 P), and 0.08g of catalyst pyridine was added dropwise, heated to 70°C, reacted for 36h, cooled after the reaction, and distilled under reduced pressure, and the product was sequentially extracted by tetrahydrofuran, dichloromethane and acetone to obtain a hydroxyl-terminated hyperbranched polyphosphazene flame retardant.
[0031] (3) The diisocyanate monomer of 3g is mixed with the polyether polyol of 5g, and the dibutyltin dilaurate of 0.1g is added dropwise, in N 2 React at 70°C for 2 hours in the atmosphere...
Embodiment 2
[0033] (1) Add 0.2 g of carboxycyclotriphosphazene to 10 mL of SOCl 2 , pass into N 2 Exhaust the air, heat to 70°C, react for 5h, distill under reduced pressure after reaction, wash the product with dichloromethane and acetone, and obtain cyclotriphosphazene acid chloride.
[0034] (2) Add 0.5% of acid chloride cyclotriphosphazene into acetonitrile solvent, stir to dissolve and then pass into N 2 Exhaust air, add 0.9g dihydroxy DOPO derivative (C 17 h 20 NO 4 P), and 0.1g of catalyst pyridine was added dropwise, heated to 85°C, reacted for 36h, cooled after the reaction, and distilled under reduced pressure, and the product was sequentially extracted by tetrahydrofuran, dichloromethane and acetone to obtain a hydroxyl-terminated hyperbranched polyphosphazene flame retardant.
[0035] (3) The diisocyanate monomer of 3.3g is mixed with the polyether polyol of 5g, and the dibutyltin dilaurate of 0.15g is added dropwise, in N 2 React at 70°C in the atmosphere for 2.5h to ob...
Embodiment 3
[0037] (1) Add 0.2 g of carboxycyclotriphosphazene to 15 mL of SOCl 2 , pass into N 2 Exhaust the air, heat to 70°C, react for 8 hours, distill under reduced pressure after the reaction, wash the product with dichloromethane and acetone, and obtain the acid chloride cyclotriphosphazene.
[0038] (2) Add 0.5% of acid chloride cyclotriphosphazene to 1,4 dioxane solvent, stir to dissolve and then pass into N 2 Exhaust the air, add 1 g dihydroxy DOPO derivative (C 17 h 20 NO 4 P), and 0.12g of catalyst pyridine was added dropwise, heated to 80°C, reacted for 48h, cooled after the reaction, and distilled under reduced pressure, and the product was sequentially extracted by tetrahydrofuran, dichloromethane and acetone to obtain a hydroxyl-terminated hyperbranched polyphosphazene flame retardant.
[0039] (3) The diisocyanate monomer of 3.5g is mixed with the polyether polyol of 5g, and the dibutyltin dilaurate of 0.2g is added dropwise, in N 2 React in the atmosphere at 75°C for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon residual rate | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com