Antiproliferative compounds and second active agents for treatment of multiple myeloma
A multiple myeloma, compound technology, applied in the direction of active ingredients of boron compounds, active ingredients of heterocyclic compounds, organic active ingredients, etc., can solve problems such as disease recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0387] Example 1: (S)-4-(4-(4-(((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindoline-4-yl) Synthesis of oxy)methyl)benzyl)piperazin-1-yl)-3-fluorobenzonitrile (compound 2)
[0388]
[0389] (4S)-5-Amino-4-(benzyloxycarbonylamino)-5-oxo-pentanoic acid tert-butyl ester. To a solution of (2S)-2-(benzyloxycarbonylamino)-5-tert-butoxy-5-oxo-pentanoic acid (150g, 445mmol) in 1,4-dioxane (1.50L) Di-tert-butyl dicarbonate (155 g, 711 mmol), pyridine (70.3 g, 889 mmol) and ammonium bicarbonate (105 g, 1.33 mol) were added. The reaction mixture was stirred at 18°C for 16 hours, then concentrated. The residue was dissolved in ethyl acetate (5.0 L) and water (5.0 L), the organic layer was separated and washed with HCl (3.0 mL, 1 N), saturated sodium bicarbonate (3.0 L), brine (3.0 L), Drying over anhydrous sodium sulfate, filtration and concentration gave crude (4S)-5-amino-4-(benzyloxycarbonylamino)-5-oxo-pentanoic acid tert-butyl ester (450 g, crude material) as white Solid, which was...
Embodiment 2
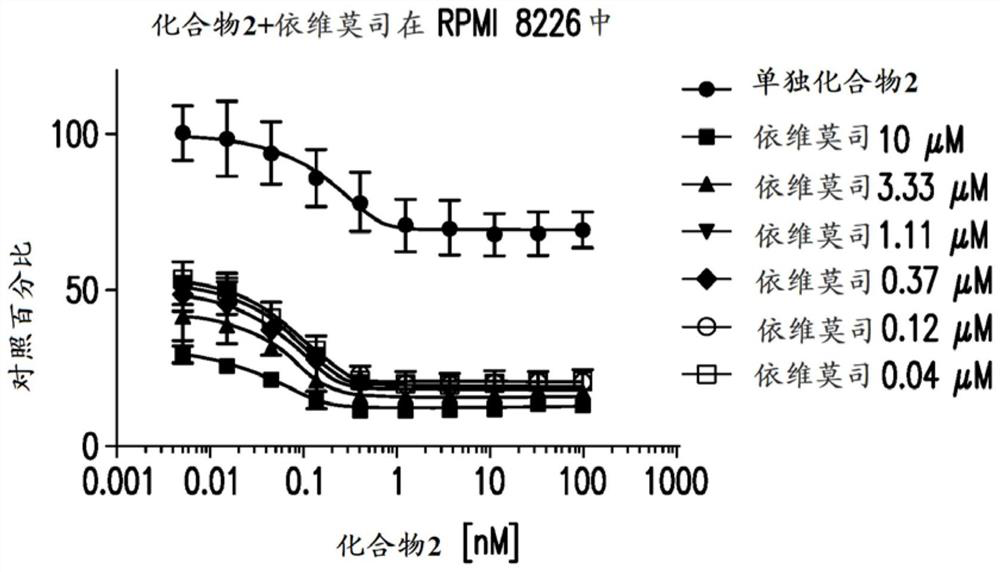
[0399] Example 2: Anti-proliferative effect on multiple myeloma
[0400] Cell culture material: Human multiple myeloma cell lines were purchased from suppliers and cultured at 37 °C with 5% CO in the medium 2 ,As shown in Table 1. Lenalidomide- and pomalidomide-resistant cell lines were obtained as previously described (Lopez-Girona et al. Leukemia 2012;26(11):2335). All cell lines were maintained in log phase, and cell density and viability were monitored by trypan blue exclusion using a Vi-cell XR cell viability analyzer (Beckman Coulter, Brea, CA).
[0401] Table 1: Multiple myeloma cell lines tested
[0402]
[0403] Preparation of test article solutions: Assuming a maximum volume of 50 μL, compounds were plated into black 384-well plates (Corning Inc.) to a final DMSO volume of 0.1%. A 10-point dose-response starting at 10 μM with a dilution ratio of 1:3 was printed in duplicate by acoustic dispense using the EDC ATS-100 platform. Alternatively, use a 10-point dose...
Embodiment 3
[0408] Example 3: Off-target effects and impact of compound 1 / compound 2
[0409] α1 adrenergic and dopamine D2 receptors. METHODS: Binding and functional assays of α1 adrenergic and dopamine D2 receptors were performed by Eurofins Cerep according to their method.
[0410] α1 adrenergic receptors. Binding at 10 μM. Binding assays evaluate the affinity of the test articles for non-selective alpha 1 adrenergic receptors in the rat cerebral cortex. Membrane homogenates of the cerebral cortex were mixed with 0.25 nM [ 3 H] Prazosin was incubated with prazosin in duplicate at room temperature for 60 minutes. After the incubation period, samples were filtered through glass fiber filters, the filters were dried, and radioactivity was counted using a scintillation counter. Results are expressed as mean percent inhibition of control radioligand binding.
[0411] combined IC 50 . To determine the binding IC of nonselective α1-adrenergic receptors 50 , with different concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com