Preparation method of chiral-magnetic hydrogel
A hydrogel and chiral technology, applied in the field of chiral-magnetic hydrogel preparation, can solve problems such as disappearance, hindering the charge transfer process of chiral materials, and weakening chirality, so as to maintain integrity, rapid preparation and The effect of simple detection and process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]The steps of this embodiment are as follows: at room temperature, 2mmol ferric chloride and 2mmol L-valine are dissolved together in 40mL glycerin / water mixed solvent (volume ratio is 1:3) and magnetically stirred until clarification; then in the above solution After adding 10mmol of urea in the solution, transfer it to a 100mL polytetrafluoroethylene-lined autoclave, set the reaction temperature in the oven to 200°C, and react at this temperature for 12h. After the reaction is completed, use deionized water to wash three times, and re- Dispersed in deionized water to obtain L-valine modified γ-Fe 2 o 3 Nanoparticle deionized aqueous solution (Fe element content is 4.2mg / mL); the synthesized L-valine-modified γ-Fe 2 o 3 Nanoparticle deionized aqueous solution and 20w% acrylamide AA, 1v% methylenebisacrylamide TMED, 1w% tetramethylethylenediamine NMBA and 5w% potassium persulfate KPS in volume ratio (AA:NMBA:TMED:KPS: NPs=10:3:3:4:5) mixed, and added to a four-way quar...
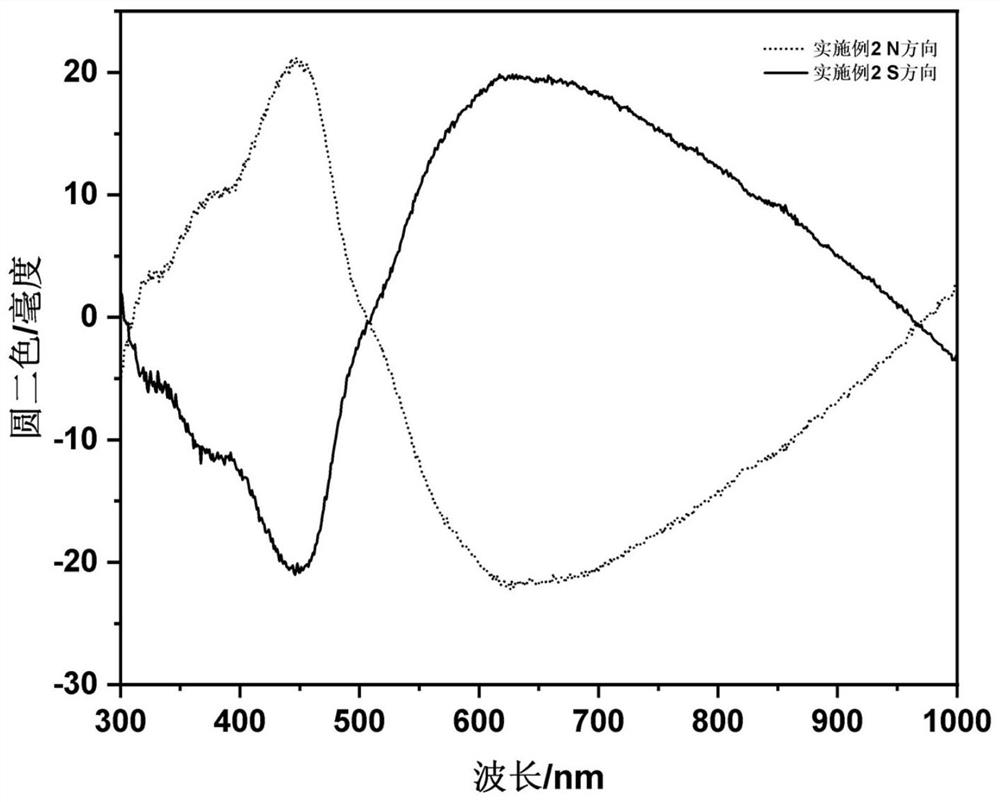
Embodiment 2
[0040] The steps of this embodiment are as follows: at room temperature, 2mmol ferric chloride and 2mmol L-valine are dissolved together in 40mL glycerin / water mixed solvent (volume ratio is 1:3) and magnetically stirred until clarification; then in the above solution After adding 10mmol of urea in the solution, transfer it to a 100mL polytetrafluoroethylene-lined autoclave, set the reaction temperature in the oven to 200°C, and react at this temperature for 12h. After the reaction is completed, use deionized water to wash three times, and re- Dispersed in deionized water to obtain L-valine modified γ-Fe 2 o 3 Nanoparticle deionized aqueous solution (Fe element content is 4.2mg / mL); the synthesized L-valine-modified γ-Fe 2 o 3 Nanoparticle deionized aqueous solution and 20w% acrylamide AA, 1v% methylenebisacrylamide TMED, 1w% tetramethylethylenediamine NMBA and 5w% potassium persulfate KPS in volume ratio (AA:NMBA:TMED:KPS: NPs=10:3:3:4:5) mixed, and added to a four-way qua...
Embodiment 3
[0042] The steps of this embodiment are as follows: 2mmol ferric chloride and 2mmol D-valine are dissolved together in 40mL glycerin / water mixed solvent (volume ratio is 1:3) at room temperature, and magnetically stirred until clarification; Add 10mmol urea to the solution and transfer it to a 100mL polytetrafluoroethylene-lined autoclave. Set the reaction temperature in the oven to 200°C and react at this temperature for 12h. After the reaction is completed, use deionized water to wash three times and re- Dispersed into deionized water; the synthesized D-valine modified γ-Fe 2 o 3 Nanoparticles (NPs) deionized aqueous solution with a concentration of 20wt.% AA, a concentration of 1vol.% TMED, a concentration of 1wt.% NMBA and a concentration of 5wt.% KPS aqueous solution by volume ratio (AA:NMBA:TMED: KPS:NPs=10:3:3:4:5) mixed, and added to a four-way quartz cuvette and reacted at room temperature for 10min; the quartz cuvette was placed in a 1T parallel magnetic field and m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com