Flusilazole hapten, antigen, antibody, detection device and detection method thereof
A flusilazole and hapten technology, which is applied in the field of antigen, antibody, detection device, and flusilazole hapten, can solve the problems of complex detection process, high cost, and failure to meet supervision and law enforcement, and achieve good specificity and easy operation , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
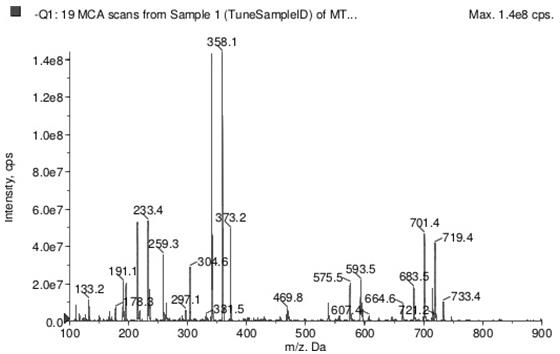
[0023] In this example, the 1.0Msolution in THF specification of 4-fluorophenylmagnesium bromide supplied by Sinopharm Chemical Reagent Co., Ltd. was selected for p-fluorophenylmagnesium bromide, and the analytical purity of cuprous cyanide was selected from Sinopharm Chemical Reagent Co., Ltd. Cuprous cyanide, chloromethyl methyl dichlorosilane select 95% (chloromethyl) methyl dichlorosilane supplied by Sinopharm Chemical Reagent Co., Ltd., and hydrochloric acid select 36%-38% supplied by Sinopharm Chemical Reagent Co., Ltd. Hydrochloric acid, ethyl acetate select analytically pure ethyl acetate supplied by Sinopharm Group Chemical Reagent Co., Ltd., anhydrous sodium sulfate select 99% anhydrous sodium sulfate supplied by Sinopharm Group Chemical Reagent Co., Ltd., 1,2,4-triazole- 97% methyl 1,2,4-triazole-3-carboxylate supplied by Sinopharm Chemical Reagent Co., Ltd., N,N-dimethylformamide selected from Sinopharm Chemical Reagent Co., Ltd. The analytically pure N,N-dimethylf...
Embodiment 2
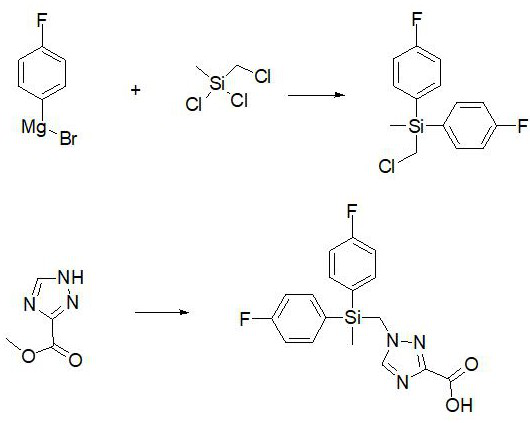
[0025] Example 2 Synthesis of flusilazole hapten for immunization
[0026] The preparation method of flusilazole hapten for immunization comprises the following steps:
[0027] S1. Take a 100ml single-neck bottle, measure 17ml of p-fluorophenylmagnesium bromide, add 9mg of cuprous cyanide, stir for 5min in an ice-water bath, add 3.9ml of chloromethylmethyldichlorosilane dropwise, and then transfer to 30°C oil Continue the reaction under the bath for 1h, then in an ice-water bath for 10min, dropwise add 5ml of 1M hydrochloric acid, stir for 0.5h, keep the ice-water bath, add an appropriate amount of distilled water and ethyl acetate, stir well, separate the liquid, dry with anhydrous sodium sulfate, concentrate, mix The first intermediate was obtained by passing a column with a filler of 200 mesh silica gel powder;
[0028] S2. Take another 100ml single-neck bottle, put all the first intermediates in a total of 2.4g, take by weighing 1.19g 1,2,4-triazole-3-carboxylate methyl e...
Embodiment 3
[0029] Example 3 Coating with flusilazole hapten synthesis
[0030] The preparation method of flusilazole hapten for coating comprises the following steps:
[0031] A1. Take a 100ml single-neck bottle, weigh 1g of flusilazole technical drug, 0.82g of sublimation sulfur, add 20ml of DMF, react in an oil bath at 156°C overnight, concentrate the reaction solution, dilute with water, extract with ethyl acetate, and dry with anhydrous sodium sulfate, Concentrate to obtain the second intermediate;
[0032] A2. Take another 100ml single-neck bottle, load all the second intermediates, weigh 1.5eq potassium carbonate, 1.2eq t-butyl 4-bromobutyrate, add 15ml DMF, react in an oil bath at 80°C for 3h, cool the reaction solution and concentrate , diluted with water, extracted with ethyl acetate, directly mixed with samples and purified by a column filled with 200-mesh silica gel powder to obtain the third intermediate. In this step, 1eq is calculated as the original drug of flusilazole; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com