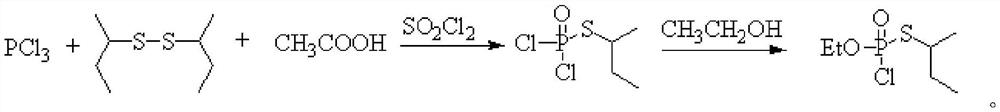
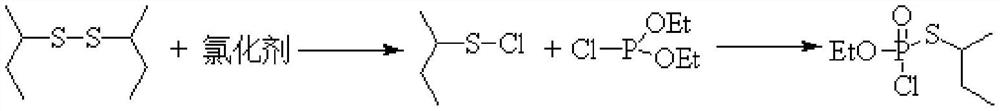Method for continuously synthesizing O-ethyl-S-sec-butyl thiophosphoryl chloride
A technology of sec-butyl phosphorothioate and di-sec-butyl disulfide is applied in the field of continuous synthesis of O-ethyl-S-sec-butyl phosphorothio chloride, which can solve the problems of low production efficiency, yield and Low purity, cumbersome operation and other problems, to achieve the effect of improving product quality, reducing reaction time, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
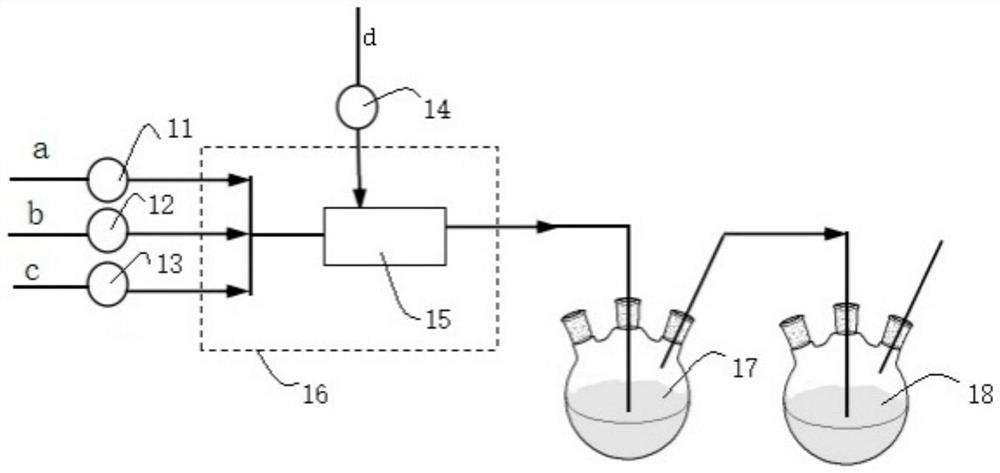
[0029] This embodiment provides a method for continuous synthesis of O-ethyl-S-sec-butylphosphoryl thiochloride, comprising the following steps:
[0030] Chlorine gas is continuously fed into the tubular reactor through a mass flow meter at a flow rate of 248.5mL / min, while toluene and di-sec-butyl disulfide are continuously fed through a metering pump at a rate of 6.0g / min and 2.0g / min, respectively. Pass into the tubular reactor, control the reaction time to be 3min, and the reaction temperature is -10°C to obtain the sulfur-based chloride intermediate. Subsequently, the sulfur-based chloride intermediate is connected to the pipe reactor at a position 5 / 9 from the inlet end. The diethyl chlorophosphite that enters is contacted, and the feeding flow rate of diethyl chlorophosphite is 3.5g / min, and the oxidation reaction is continued, and the reaction time is controlled to be 2.5min. The product is collected in a product collection bottle, and the solvent is evaporated , in O-...
Embodiment 2
[0032] This embodiment provides a method for continuous synthesis of O-ethyl-S-sec-butylphosphoryl thiochloride, comprising the following steps:
[0033] Chlorine gas is continuously fed into the tubular reactor through a mass flow meter at a flow rate of 273.4mL / min, while toluene and di-sec-butyl disulfide are continuously fed through a metering pump at a rate of 12.0g / min and 2.0g / min, respectively. Pass into the tubular reactor, control the reaction time to be 2.0min, and the reaction temperature is -5°C to obtain the sulfur-based chloride intermediate. Subsequently, the sulfur-based chloride intermediate is passed through the pipe reactor at a position 1 / 2 from the inlet end. The diethyl chlorophosphite that enters is contacted, and the feeding flow rate of diethyl chlorophosphite is 3.5g / min, continues to carry out oxidation reaction, and control reaction time is 1.8min, and product is collected with product collection bottle, evaporates solvent , in O-ethyl-S-sec-butyl ...
Embodiment 3
[0035] This embodiment provides a method for continuous synthesis of O-ethyl-S-sec-butylphosphoryl thiochloride, comprising the following steps:
[0036] Chlorine gas is continuously fed into the tubular reactor through a mass flow meter at a flow rate of 621.3mL / min, while toluene and di-sec-butyl disulfide are continuously fed through a metering pump at a rate of 40.0g / min and 5.0g / min, respectively. Pass into the tubular reactor, control the reaction time to be 2min, and the reaction temperature is 5°C to obtain the sulfur-based chloride intermediate, and subsequently, the sulfur-based chloride intermediate is passed into the tubular reactor at a position 4 / 7 from the inlet end Diethyl chlorophosphite contacts, and the feeding flow rate of diethyl chlorophosphite is 9.2g / min, continues to carry out oxidation reaction, and the control reaction time is 1.5min, and the product is collected with product collection bottle, evaporates and removes solvent, obtains O-ethyl-S-sec-bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com