Cistanche pill production process, product and quality control method thereof
A quality control method, Cistanche pill technology, applied in the direction of pill delivery, medical preparations containing active ingredients, anti-inflammatory agents, etc., can solve the problem of inability to ensure the effect and quality, unable to fully and truly reflect the quality of Cistanche medicinal materials, and lack of simplicity It is easy to mass produce Cistanche medicinal materials, such as the method of quality standard control, to achieve the effect of avoiding water absorption and deterioration, and the production process is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
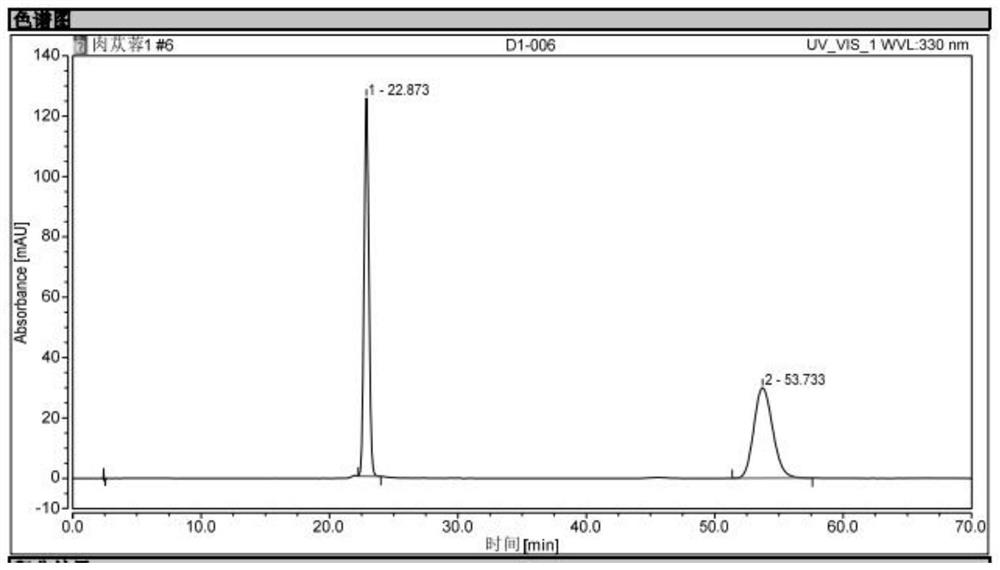
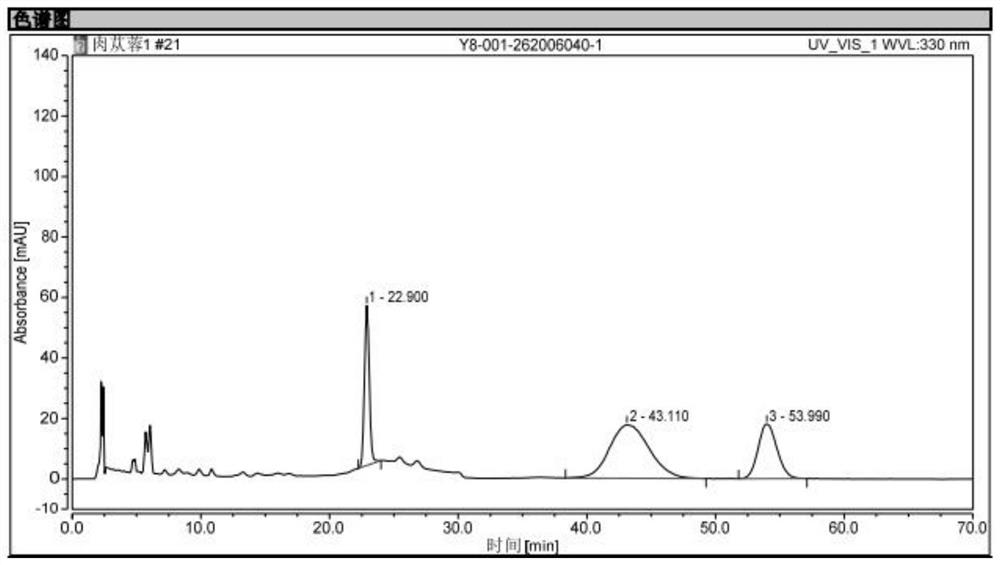
[0050] Preparation of reference substance solution: take echinacoside and verbascoside to obtain a mixed reference substance, add methanol with a concentration of 50% to the mixed reference substance to form a mixed solution, and obtain a reference substance solution, wherein echinacoside and verbascoside The volume concentration of glycosides is 0.2mg / ml;
[0051] Preparation of the test solution: take 1g of Herba Cistanche, put it in a 100ml brown measuring bottle, add 50ml of methanol with a concentration of 50%, weigh it after shaking well, soak it for 30min, and ultrasonicate it under 250W power and 35kHz frequency After cooling for 40 minutes, weigh again, add methanol with a concentration of 50% to the brown measuring bottle to make up the weight lost by ultrasound, shake well, let it stand still, take the supernatant, filter, and take the subsequent filtrate to obtain the test product. product solution;
[0052] Determination of the content of echinacoside and verbasc...
Embodiment 1
[0061] A kind of production technology of cistanche pill, comprises the following steps:
[0062] Cistanche was washed and soaked in water under the following conditions: vacuum degree of 0.12MPa, temperature of 70°C, time of 40min, and then cut into 3mm slices to obtain Cistanche slices;
[0063] After pulverizing the cistanche tablets, pass through a 110-mesh sieve, and then mix for 60 minutes to obtain the medicinal powder;
[0064] After adding water to the above-mentioned medicinal powder, it is made into pellets;
[0065] The above-mentioned pellets are dried at 70° C. until the moisture content is 5%, and the finished Cistanche pills are obtained.
[0066] Carry out quality control to above-mentioned cistanche pill, comprise following detection item:
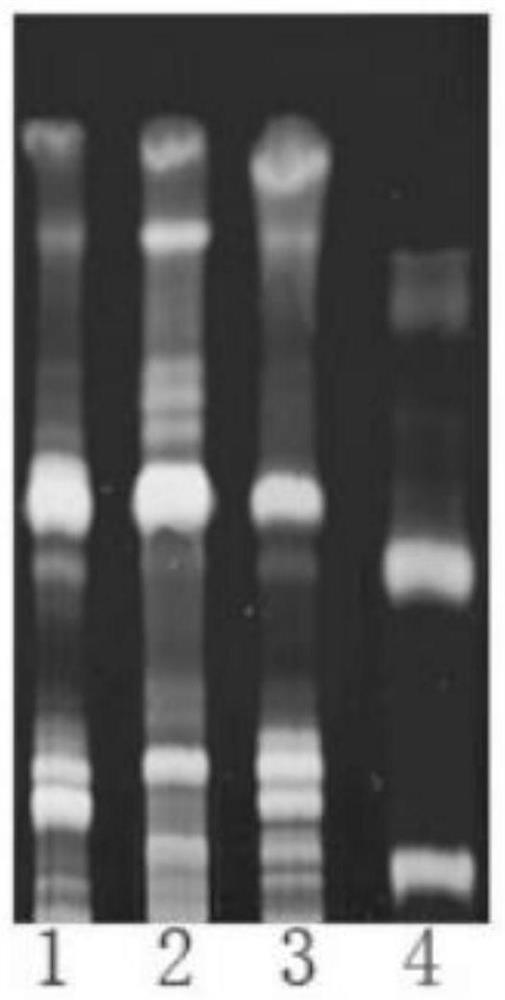
[0067] 1. Using echinacoside and verbascoside as index components, conduct a qualitative analysis of Cistanche pills by thin-layer chromatography. The specific steps are:
[0068] Take 1 g of Herba Cistanche pill, grin...
Embodiment 2
[0100] A kind of production technology of cistanche pill, comprises the following steps:
[0101] Cistanche was washed and soaked in water under the following conditions: a vacuum of 0.08 MPa, a temperature of 60°C, and a time of 30 minutes, and then cut into 3mm slices to obtain Cistanche slices;
[0102]After pulverizing the cistanche tablets, pass through a 100-mesh sieve, and then mix for 55 minutes to obtain the medicinal powder;
[0103] After adding water to the above-mentioned medicinal powder, it is made into pellets;
[0104] The above-mentioned pellets are dried at 60° C. until the moisture content is 7%, and the finished Cistanche pills are obtained.
[0105] Adopt the quality control method of embodiment 1 to detect the cistanche pill prepared in the present embodiment, and the detection result is a qualified product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com